Abstract
Microplastics can promote microbial colonisation and biofilm growth, thus being referred to as “plastispheres”. The global plastic pollution surge is likely to adversely impact ecology and human health by providing a novel habitat for microbial communities. Even though microplastics in marine environments have been the subject of in-depth research, plastispheres have recently received attention. Thus, the current study investigates the prevalence and distribution of plastispheres along the Maharashtra coast of India, considering their plausible implications for ecology and human health. Microplastics were isolated from sediment and water samples obtained from 10 sampling sites. Subsequently, these microplastic particles were subjected to ATR-FTIR and scanning electron microscopy (SEM) analyses to ascertain their chemical composition, surface topography, and presence of attached biofilms. The predominant polymers composing the microplastic particles were polypropylene (42.8%), polyethylene (28.6%), polystyrene (14.3%), and polyvinyl chloride (14.3%). SEM analysis revealed the presence of topographical structures and degradation effects, facilitating microbial attachment on the microplastic surface. About 50% of the microplastic particles tested positive for biofilms, with over 66% of those collected from Girgaon and Malvan beaches exhibiting biofilm presence. These positively screened particles also displayed comparatively rough surface structures, likely enhancing microbial colonisation. Microplastic ageing and polymer type could positively affect microbial colonisation. Diatoms and fungal hyphae exhibit varied interactions with microplastic polymers. Notably, microplastics host various reproductive stages of fungi, as evidenced by filamentous networks, mycelia, and conidiophores.
Similar content being viewed by others
Introduction
High and low-density polyethylene (HDPE and LDPE), polypropylene (PP), polyvinyl chloride (PVC), and polystyrene (PS) are among the widely manufactured thermoplastics (Sánchez 2020; Zhang et al. 2021). These versatile thermoplastics are used in a wide range of daily items, playing a significant role in the notable rise of worldwide plastic production, which presently amounts to 368 million metric tons annually (Sooriyakumar et al. 2022). However, the surge in plastic production and extensive utilisation has led to a parallel escalation in releasing plastic waste into the environment (Geyer et al. 2017). Microplastics (plastic less than 5 mm) pollution is an emerging environmental concern (Thompson et al. 2004). Microplastics are classified as either primary or secondary. Primary microplastics originate from sources with an initial size of less than 5 mm, while secondary microplastics result from the breakdown of larger plastic fragments (Dong et al. 2021). These tiny plastic particles have become ubiquitous in terrestrial, freshwater, estuarine, marine, polar, and remote regions (Dong et al. 2021). Given their small size, various aquatic organisms, mainly marine fauna, readily ingest microplastics, leading to their potential accumulation and dispersion throughout the food chain (Roman et al. 2021). Numerous studies have extensively documented the adverse impacts of microplastics on a diverse array of organisms, spanning from zooplankton to molluscs, fish, seabirds, turtles, and mammals (Zhang et al. 2021). Moreover, the hydrophobic nature and the high surface area-to-volume ratio of microplastics facilitate the accumulation of various contaminants, including heavy metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, perfluorinated alkyl substances, polybrominated diphenyl ethers, and pharmaceuticals and personal care products (PPCPs) (Bakir et al. 2014a; Caruso 2019; Atugoda et al. 2021; Khalid et al. 2021). The potential of microplastics to act as carriers for a range of contaminants could pose additional threats to ecosystems, wildlife, and potentially human health (Bakir et al. 2014b; Atugoda et al. 2021; Khalid et al. 2021).
Besides serving as vectors for various pollutants, the interaction between microplastics and microorganisms has recently become a topic of great interest (Dong et al. 2021; Stenger et al. 2021; Sooriyakumar et al. 2022). Microorganisms can attach to the surface of microplastics and form biofilms within a relatively short time (Harrison et al. 2014; Khatoon et al. 2018). For instance, studies have shown that bacterial communities quickly colonised LDPE when exposed to coastal sediments, demonstrating the speed of this process within just a week (Harrison et al. 2014). Various factors, including surface texture, hydrophobicity, and the presence of chemical additives, have been identified as influencing the formation of biofilms on the surface of microplastics (Tu et al. 2020; Wang et al. 2021a, b; Sooriyakumar et al. 2022). According to Sooriyakumar et al. (2022), the surface characteristics of microplastics inherently promote microbial colonisation. Microplastics featuring irregular or rough surfaces serve as attachment points for microorganisms, creating microenvironments conducive to microbial colonisation and the development of biofilms. Similarly, the hydrophobic nature and smaller particle size of microplastics, which offer a larger surface area relative to their volume (Atugoda et al. 2021), provide additional sites for microbial attachment.
Biofilms encompass a variety of microorganisms existing in symbiotic collaboration (Sooriyakumar et al. 2022). Among the microorganisms affiliated with microplastics, autotrophs like photosynthetic bacteria and algae can independently produce their own sustenance. Conversely, heterotrophs depend on the surplus food generated by autotrophs during their coexistence (Bolan et al. 2020). Consequently, microplastics offer a distinctive habitat for microorganisms and have been denoted as “plastispheres” (Zettler et al. 2013). Plastispheres signify a novel ecological niche that can have significant environmental implications. As the research on microplastics and their interactions with microorganisms grows, our understanding of the ecological consequences and potential impacts on marine ecosystems will become more comprehensive. Biofilm formation can modify the physical and chemical attributes of microplastics, influencing their degradation and dispersion in the water column (sinking and buoyancy rate), their capacity to adsorb and transport various contaminants, and ultimately the trophic transfer and environmental release of adsorbed chemicals (Rummel et al. 2017; Stabnikova et al. 2021; Stenger et al. 2021; Vaseashta et al. 2021; Zhang et al. 2021). It has been established that a variety of microbial communities, such as toxic, pathogenic, invasive, or plastic-degrading species, can be found in biofilms on microplastics (Zettler et al. 2013; McCormick et al. 2014; Oberbeckmann et al. 2014; Curren and Leong 2019; Li et al. 2019; Zhang et al. 2021). These potentially harmful microorganisms might be extensively disseminated in seawater, shielded by biofilms, endangering human health (Metcalf et al. 2022a). A plethora of investigations have been carried out regarding microplastics in marine environments, addressing diverse topics (Ajith et al. 2020; Gola et al. 2021; Ahmed et al. 2021; Perumal et al. 2022; Biswas and Pal 2023), including their function as transporters for various contaminants (Bakir et al. 2014a; Caruso 2019; Atugoda et al. 2021; Khalid et al. 2021; Kumkar et al. 2023). However, recent focus has shifted towards a more specific examination of biofilms associated with microplastics (Kumar et al. 2022a; Kaur et al. 2022). Furthermore, previous research has indicated that obtaining a more profound understanding of the processes involved in biofilm formation on microplastic surfaces necessitates more detailed insights into the plastisphere compared to naturally occurring substrate-associated aggregates, such as microbial communities on cellulose, wood, and glass (Sooriyakumar et al. 2022).
The projected amount of plastic debris in the Earth’s oceans is expected to reach 5.25 trillion particles, with microplastics comprising 92%. Approximately 80% of these plastic particles are linked to terrestrial sources (Coyle et al. 2020; Cincinelli et al. 2021; Gola et al. 2021). Notably, the benthic region of the Indian Ocean is reported to have the highest prevalence of microplastic contamination, quantified at 4 billion fibres per square kilometre (Eriksen et al. 2014; Woodall et al. 2014). Thorough examinations of microplastic occurrence within sedimentary substrates have been extensively documented across various coastal regions of India, including Goa (Veerasingam et al. 2016), Tamil Nadu (Karthik et al. 2018), Karnataka (Tiwari et al. 2019), Kerala (Robin et al. 2020), Odisha (Patchaiyappan et al. 2021), Andhra Pradesh (Sambandam et al. 2022), West Bengal (Kumar et al. 2022b), the Andaman and Nicobar Islands (Goswami et al. 2020; Patchaiyappan et al. 2021), and Maharashtra (Kumkar et al. 2023; Tiwari et al. 2019). However, there is currently a research gap concerning biofilms’ presence and prevalence on microplastics along the Maharashtra coast. Moreover, we speculate that significant differences in biofilm density on microplastics obtained from sediment and water samples along the Maharashtra coast are likely due to the region’s diverse demographic zones, human activities, and land use patterns. Additionally, the diversity in microplastic origins offers the potential to gain insights into the relationship between microbial diversity residing on the surfaces of different microplastic polymers and the geographical locations from which they are sourced. To address these gaps, our study pursues the following objectives: (1) Collection and examination of microplastics obtained from coastal sediment and water along the Maharashtra coast to discern their surface morphology and disintegration patterns, (2) To identify the microbial communities associated with the microplastics, (3) Characterisation of the microplastics and investigation into the interplay between the diversity of microbial life inhabiting on the surfaces of microplastic polymer types and the specific regions of their origin.
Materials and Methods
Study Locations
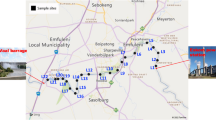
Maharashtra, ranked as the second most populous state and covering the third-largest geographical area in India, is bounded by the Arabian Sea on one side and the Western Ghats Mountain ranges on the other. The state encompasses five coastal districts: Thane, Raigad, Mumbai, Ratnagiri, and Sindhudurg. Plastic pollution from anthropogenic activities poses a significant challenge along the Maharashtra coast, with major contributors including densely populated regions, urban development, industrial zones, fishing ports, and coastal tourism (Kumkar et al. 2023). In our investigation, we deliberately chose ten distinct sample sites labelled S1 to S10, strategically positioned across the five coastal districts mentioned above, thus providing a representative overview of the entire Maharashtra coastline (Fig. 1a).
(a) Geographical distribution of the Maharashtra coastline showing collection sites (n = 10); pie chart within the map of Maharashtra state indicates overall biofilm-positive and negative sites; (b and c) Percent distribution of biofilm-positive sites in sediment and coastal water, respectively; (d) Site-specific distribution of biofilm-positive microplastics (“#” and “*” indicates biofilm found on microplastics isolated from coastal sediment and water samples, respectively)
Sampling, Microplastic Isolation and Characterisation
In August 2021, we gathered beach sediment and surface water samples from ten locations along the Maharashtra coast, following the methodology outlined by Karthik et al. (2018) and Robin et al. (2020). A slight alteration was made to the procedure, considering our particular emphasis on investigating biofilms on microplastic particles. This modification was implemented to avoid the potential detachment of biofilms from microplastics during sample processing and extraction, which chemical interactions could influence. Stainless steel scoops were employed at each assigned sampling location to collect the uppermost 5 cm of beach sediment. Sediment samples were acquired from three quadrats (30 cm x 30 cm) on each beach, spaced 100 m apart. The sediment was placed in stainless-steel containers and sieved through a 5-mm mesh to remove particles larger than 5 mm. Likewise, 100 L of water were gathered from a depth of 0 to 25 cm below the surface for water samples, utilising a 5 L stainless-steel sampling container. The obtained water was subsequently strained through a stainless-steel sieve with a mesh size of 100 μm.
The sieves underwent thorough inspection, and microplastic particles ranging from 3 to 5 mm in size were manually separated from the sediment and water samples using sterile forceps. These isolated particles were then individually placed into glass vials for subsequent analysis. The focus on choosing larger microplastic particles was intentional in clearly separating them from the samples. This helps to minimise the chance of unintentionally including microplastics from outside sources. In an aseptic laboratory environment, microplastic particles from each sample location were pooled together. Six particles were randomly chosen from this combined microplastic pool for each sampling location—three from water samples and three from sediment samples. To maintain an unbiased selection of microplastic particles, an anonymous individual, unaware of the sample sites or any sampling data, made the particle selections. The glass vials containing the microplastic particles were tightly sealed and covered with aluminium foil to prevent contamination from external sources. The task of impartially selecting microplastic particles was meticulously carried out in a strictly aseptic environment.
The selected microplastic particles were thoroughly examined for their colour (red, green, blue, white, yellow, and black) and morphological characteristics using the Olympus SZ61 stereo zoom microscope. To identify the polymeric composition of the microplastics and determine their potential source, we employed Attenuated Total Reflectance Fourier-transform Infrared Spectroscopy (ATR-FTIR) (Ballent et al. 2016). Following ATR-FTIR analysis using the Thermo Scientific NicoletTM iS20, the spectrum data of the microplastic particles were compared to the OMNiC reference library.
Detection of Biofilms on Microplastics
We employed a high-resolution imaging method to comprehensively understand microplastic surface characteristics, biofilm formation, and changes induced by degradation. The goal was to explore microbial communities and uncover the importance of surface texture and topography in biofilm development. Scanning Electron Microscopy (SEM) was chosen for its effectiveness in revealing surface texture, topographical features, and attached materials on microplastic surfaces, including microorganisms like bacteria, fungi, and algae (Kumkar et al. 2021; Verma et al. 2022). This technique plays a crucial role in unveiling the complexities of microplastic disintegration due to weathering and ageing and understanding its potential role in carrying other contaminant pollutants. In our study, six microplastic particles, including three from sediment and three from water samples at each designated station, underwent preparation for SEM analysis. This preparation involved sputter-coating the particles with a thin layer of gold and imaging using a Scanning Electron Microscope (Tescan Mira3, Tescan Orsay Holding, Brno, Czech Republic).
Data and Image Analysis
All values that are given as %, mean ± standard error (M ± SE), or otherwise specified. PAST freeware Version 4.03 (Hammer et al. 2001) was used for the data analysis.
Results and Discussion
The present research contributes to our comprehension of the prevalence and distribution of biofilm formation on microplastics along the Maharashtra Coast, shedding light on the interactions between microplastics and microbes. These microbial communities, consisting of autotrophs, photosynthetic algae, fungi, and bacteria, form biofilms and exhibit rapid growth on the solid surfaces of microplastics (Bolan et al. 2020; Sooriyakumar et al. 2022). Upon scrutinising microplastic samples from the ten sampling sites, our investigation unveiled the presence of biofilms on microplastic particles collected from half of these sites (50%) (Fig. 1a). Among the samples positive for biofilms, 30% were derived from sediment while 20% were obtained from coastal water samples (Fig. 1b and c). Microplastic particles from sampling sites S1, S3, S5, S7, and S10 were identified as biofilm-positive (Fig. 1d). Among the designated sampling stations, the Girgaon (S3) and Malvan (S10) locations displayed the highest percentage (66.66%) of biofilm positive microplastic (Fig. 1d). In contrast, the Tarapur (S1), Diveagar (S5), and Guhagar (S7) stations had 33.33% of microplastic particles exhibiting biofilm (Fig. 1d). The formation of biofilms and the degree of microbial colonisation can be significantly influenced by various factors, including surface roughness, polymer composition, hydrophilic or hydrophobic characteristics, and other relevant attributes (Sooriyakumar et al. 2022). While microplastics themselves lack direct porosity, their surfaces can develop pits, fissures, and cavities through weathering, abrasion, and photo-oxidation processes (Atugoda et al. 2021; Kumkar et al. 2021, 2023). The presence of such morphological features, including flakes, eroded surfaces, trenches, and cavities, not only facilitates the adsorption of pollutants like heavy metals (Turner and Holmes 2011; Li et al. 2018; Shruti et al. 2019; Atugoda et al. 2021; Khalid et al. 2021; Kumkar et al. 2021, 2023) but also encourages the formation of biofilms (Tu et al. 2020; Wang et al. 2021a, b; Sooriyakumar et al. 2022). Using detailed SEM analysis, Tiwari et al. (2019) demonstrated that microplastics collected from Girgaon exhibit rougher surfaces than those from other places. Our present data align with this past trend, revealing a noticeable pattern of increased biofilm colonisation on microplastic particles with rough surfaces. We observed more microplastic particles with positive biofilm presence in samples collected from Girgaon and Malvan than in other locations. This is likely associated with the rougher surfaces of the microplastics obtained from these specific areas. This holds particular significance as both microplastics and the pollutants they absorb can accumulate in substantial quantities in the environment, potentially exerting adverse effects on ecosystems and food chains. Verdú et al. (2023) showed that biofilm growth considerably affects the vector transport of personal care products such as triclosan-adhered polyethylene microplastics into Daphna magna. Furthermore, Guan et al. (2020) conclusively proved that biofilm formation increased microplastics’ function in the transport of trace metals, such as nickel (Ni), copper (Cu), zinc (Zn), and cadmium (Cd) in the aquatic environment. In a recent study by Kumkar et al. (2023), various emerging pollutants were identified in the coastal waters of Maharashtra. These pollutants included heavy metals such as vanadium (V), copper (Cu), arsenic (As), nickel (Ni), cobalt (Co), chromium (Cr), cadmium (Cd), and lead (Pb). Additionally, pharmaceuticals and personal care products such as metoprolol, tramadol, venlafaxine, triclosan, bisphenol A, and bisphenol S were detected, along with plasticisers like di-n-butyl phthalate (DBP) and bis(2-ethylhexyl) phthalate (B2EHP or DEHP). Moreover, earlier investigations have showed weathered microplastic particles’ capability to carry these pollutants into fish (Kumkar et al. 2021, 2023; Verma et al. 2022). Considering that fish and fish-related products constitute a primary food source for the population residing in the coastal regions of Maharashtra, the presence of diverse pollutants along the Maharashtra Coast (Kumkar et al. 2023), the transport capacity of microplastics for these pollutants (Li et al. 2018; Kumkar et al. 2021, 2023), and the potential enhancement of microplastics uptake by marine organisms through biofilms (Fabra et al. 2021), pose a substantial threat to aquatic biota and human health.
Among the five distinct morphological categories considered (Supplement 1a), only microplastic fragments and beads exhibited positive biofilm presence (Fig. 2a). In this context, microplastic fragments were notably prevalent, constituting 85.7% of instances with associated biofilms, while beads accounted for 14.3%. Regarding colour, white microplastics showed the highest occurrence of biofilm, making up 28.5% of the total instances, followed by other colour categories, each representing 14.3% (Fig. 2b). Notably, white-coloured microplastics have often been observed in the digestive systems of marine fish (Tanaka and Takada 2016; Pan et al. 2021). Previous research has investigated the impact of microplastic colour and biofilm growth on the ingestion and susceptibility of species to such particles (Peters and Bratton 2016; Ory et al. 2017; Naidoo et al. 2020; Kumkar et al. 2021). This study analysed the polymeric composition of 60 microplastic particles using ATR-FTIR. Spectral analysis (Supplement 1b) revealed the following polymers: polypropylene (PP), polyethylene (PE), polystyrene (PS), and polyvinyl chloride (PVC). The per cent distribution of biofilm-positive microplastic polymers along the Maharashtra coast is as follows: PP (42.8%), PE (28.6%), PS (14.3%), and PVC (14.3%), respectively (Fig. 2c). Over 70% of the polymers identified in microplastic particles consisted of PE and PP. This finding is reasonable, considering that the study sites were either urban (Urban area) or suburban to rural (Tourism) areas, where disposable items like food wrappers, straws, water and beverage bottles, wire insulation, plastic bags, and sachets for personal care products (shampoo, conditioners, and scrub) are commonly utilised. It is crucial to highlight that PP, PE, PS, and PVC have demonstrated a higher affinity for a range of environmental contaminants, including heavy metals and hydrophilic and/or hydrophobic chemicals (Amelia et al. 2021). Specifically, PE exhibits the highest affinity for heavy metals, while PS shows the highest affinity for hydrophilic and hydrophobic chemicals (Amelia et al. 2021). In this context, the presence of biofilm on white microplastics in the study area, coupled with the prevalence of polymer types exhibiting a high affinity for pollutants, presents a dual threat to marine life, raising concerns.
Plastispheres are recognised for fostering the growth of biofilms, hosting diverse microbial communities (Oberbeckmann et al. 2015; Frère et al. 2018; Debroy et al. 2022; Miao et al. 2021). It is crucial to emphasise the progression from biofilm formation to subsequent biodegradation processes, leading to the deterioration of the polymer’s physical structure (Debroy et al. 2022). In our study, all plastispheres exhibited rough surfaces characterised by cracks, fissures, and trenches (Fig. 3a–d). This highlights the complex interplay among microplastics, biofilms, and polymer integrity, contributing to our understanding of their ecological implications. According to Eich et al. (2015), diatoms constitute a significant element of marine biofilms and play a crucial role in their formation and activity. SEM micrographs offer tangible evidence of the substantial colonisation of plastic surfaces by diatoms (Fig. 3a and b) and fungal colonisation (Fig. 3c and d) in the majority of the examined samples. Diatoms were prevalent on microplastic particles obtained from coastal waters of Maharashtra, while fungi (fungal hyphae or spores) were observed to have colonised 60% of the microplastic particles from sediment samples. Similar findings were reported by Eich et al. (2015), where floating plastic exhibited an increased abundance of diatoms. Hence, it is evident that microplastics can act as selective artificial microhabitats, attracting distinct microbial communities.
Surface characteristics and microbial colonisation of microplastic particles observed using SEM: (a and b) Microplastic displaying a rough surface with evident cracks, crevices, and holes (white arrowheads), hosting diatom colonisation (red arrow); (c and d) Microplastic featuring fungal colonisation, showcasing intricate mycelial networks comprising hyphal filaments (1), clusters of conidia (2), compact mycelia (3), and conidiophore structures (4)
Though fungal pathogens represent one of the most diverse microbial groups and exhibit a propensity to adhere to plastics, colonising and persisting on plastic pollutants in the environment and posing a potential pathogenic threat, their significance has only recently gained attention from the scientific community (Gkoutselis et al. 2021; Akinbobola et al. 2024). For instance, A recent investigation by Akinbobola et al. (2024) revealed that biofilms linked with plastic could function as a unique reservoir facilitating the environmental persistence of the multi-drug resistant fungal pathogen Candida auris for up to 30 days, whether in freshwater or marine water. Additionally, C. auris could transfer from plastic beads to beach sand while retaining its pathogenic properties. In the current investigation, the presence of a substantial layer of extracellular polymeric substances (EPS) forming a biofilm is apparent on the plastisphere, along with distinct filamentous fungal structures such as vegetative and reproductive hyphae (Fig. 3c and d). As noted by Priyadarshanee and Das (2023), EPS consists of proteins, nucleic acids, and carbohydrates that are recognised for promoting microbial growth and keeping them clustered in the biofilm. Abundant conidia, the asexual fungal spores, are observed surrounding the fungal hyphae (Fig. 3d). Additionally, there were compact mycelia (Fig. 3d) and fungal hyphae distributed across the surface or forming filamentous networks. The presence of conidiogenous hyphae (conidiophore) was also noteworthy, indicating efficient fungal growth and reproduction within the plastisphere (Fig. 3d). Our results support an earlier study conducted on soil samples obtained from disposal areas within the city confines of Siaya, Western Kenya (Gkoutselis et al. 2021). These sites receive a significant influx of plastic waste and sewage water from both terrestrial and river sources. While the specific identification of the fungus was not possible in our study, it is reasonable to assume the potential presence of opportunistic human pathogenic fungi in these environments. Therefore, we suggest that future studies should focus on isolating and characterising the microbial community associated with plastispheres, particularly multi-drug resistant pathogens capable of enduring transitions across diverse environmental matrices, thereby possessing the potential for widespread dissemination within the landscape (Junaid et al. 2022a, b; Metcalf et al. 2022b; Khare et al. 2024). This would assist in reducing the possible risk imposed by these biofilms-associated microorganisms on human health.
Earlier studies have indicated that the microbial colonisation of microplastics is influenced by the type of polymer (Oberbeckmann et al. 2015; Frère et al. 2018). For example, marine bacteria exhibit a preference for polystyrene (PS) polymer, while algal microbes tend to favor polyvinyl chloride (PVC) surfaces (Eich et al. 2015; Miao et al. 2021), and diatoms are more prevalent on PS foam (Carson et al. 2013). In a previous investigation, Frère et al. (2018) found that microbial communities on polyethylene (PE) were more diverse than those on PS and polypropylene (PP). However, our observations revealed more variability, such as the colonisation of fungal hyphae and diatoms on the surface of PP, while PE and PS showed predominant colonisation by fungal hyphae and diatoms, respectively (Fig. 2a and d). However, more investigation is required to fully comprehend the processes driving the preferential colonisation of microorganisms on a certain kind of microplastic polymer.
Conclusions and Future Prospective
The results of the current study indicate that microplastics create distinct microenvironments that support a diverse range of microorganisms in the marine ecosystem, including diatoms and fungal species. The ageing of microplastics enhances microbial colonisation, and the type of microplastic polymer influences microbial colonisation patterns. Diatoms and fungal hyphae display diverse interactions with microplastic polymers. Notably, microplastics are hosts for various fungi reproductive stages, as evidenced by filamentous networks, mycelia, and conidiophores. However, the presence of potentially harmful bacteria and fungi within these biofilms necessitates further investigation. The prevalence of microplastics as microbial hosts may facilitate disease transmission, posing increased risks to human health and wildlife. Our study highlights the intricate relationship between microplastics and marine microorganisms, emphasising potential implications for ecosystems, human health, and animal welfare. Further research is imperative to fully understand these interactions and develop strategies to mitigate their adverse effects. Future investigations should prioritise three key areas: (1) employing molecular techniques like metagenomics to identify the microbes residing on microplastics; (2) assessing the ecological and epidemiological impacts of the plastisphere, and (3) investigating the influence of microplastic polymer type and morphology on biofilm formation and uptake by marine organisms.
Data Availability
No datasets were generated or analysed during the current study.
References
Ahmed MB, Rahman MS, Alom J, Hasan MS, Johir MAH, Mondal MIH, Lee DY, Park J, Zhou JL, Yoon MH (2021) Microplastic particles in the aquatic environment: a systematic review. Sci Total Environ 775:145793. https://doi.org/10.1016/j.scitotenv.2021.145793
Ajith N, Arumugam S, Parthasarathy S, Manupoori S, Janakiraman S (2020) Global distribution of microplastics and its impact on marine environment—a review. Environ Sci Pollut Res 27:25970–25986. https://doi.org/10.1007/s11356-020-09015-5
Akinbobola A, Kean R, Quilliam RS (2024) Plastic pollution as a novel reservoir for the environmental survival of the drug resistant fungal pathogen Candida Auris. Mar Pollut Bull 198:115841. https://doi.org/10.1016/j.marpolbul.2023.115841
Amelia TSM, Khalik WMAWM, Ong MC, Shao YT, Pan HJ, Bhubalan K (2021) Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog Earth Planet Sci 8:1–26. https://doi.org/10.1186/s40645-020-00405-4
Atugoda T, Vithanage M, Wijesekara H, Bolan N, Sarmah AK, Bank MS, You S, Ok YS (2021) Interactions between microplastics, pharmaceuticals and personal care products: implications for vector transport. Environ Int 149:106367. https://doi.org/10.1016/j.envint.2020.106367
Bakir A, Rowland SJ, Thompson RC (2014a) Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ Pollut 185:16–23. https://doi.org/10.1016/j.envpol.2013.10.007
Bakir A, Rowland SJ, Thompson RC (2014b) Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar Coast Shelf Sci 140:14–21. https://doi.org/10.1016/j.ecss.2014.01.004
Ballent A, Corcoran PL, Madden O, Helm PA, Longstaffe FJ (2016) Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull 110:383–395. https://doi.org/10.1016/j.marpolbul.2016.06.037
Biswas T, Pal SC (2023) Emerging threats of microplastics on marine environment: a critical review of toxicity measurement, policy practice gap and future research direction. J Clean Prod 434:139941. https://doi.org/10.1016/j.jclepro.2023.139941
Bolan NS, Kirkham MB, Ravindran B, Kumar A, Ding W (2020) Microbial plastisphere: microbial habitation of particulate plastics in terrestrial and aquatic environments. Particulate plastics in terrestrial and aquatic environments. CRC, Boca Raton, pp 135–145
Carson HS, Nerheim MS, Carroll KA, Eriksen M (2013) The plastic-associated microorganisms of the North Pacific Gyre. Mar Pollut Bull 75:126–132. https://doi.org/10.1016/j.marpolbul.2013.07.054
Caruso G (2019) Microplastics as vectors of contaminants. Mar Pollut Bull 146:921–924. https://doi.org/10.1016/j.marpolbul.2019.07.052
Cincinelli A, Scopetani C, Chelazzi D, Martellini T, Pogojeva M, Slobodnik J (2021) Microplastics in the Black Sea sediments. Sci Total Environ 760:143898. https://doi.org/10.1016/j.scitotenv.2020.143898
Coyle R, Hardiman G, O’Driscoll K (2020) Microplastics in the marine environment: a review of their sources, distribution processes, uptake and exchange in ecosystems. Case Stud. Chem Environ Eng 2:100010. https://doi.org/10.1016/j.cscee.2020.100010
Curren E, Leong SCY (2019) Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci Total Environ 655:313–320. https://doi.org/10.1016/j.scitotenv.2018.11.250
Debroy A, George N, Mukherjee G (2022) Role of biofilms in the degradation of microplastics in aquatic environments. J Chem Technol Biotechnol 97:3271–3282. https://doi.org/10.1002/jctb.6978
Dong X, Zhu L, Jiang P, Wang X, Liu K, Li C, Li D (2021) Seasonal biofilm formation on floating microplastics in coastal waters of intensified mariculture area. Mar Pollut Bull 171:112914. https://doi.org/10.1016/j.marpolbul.2021.112914
Eich A, Mildenberger T, Laforsch C, Weber M (2015) Biofilm and diatom succession on polyethylene (PE) and biodegradable plastic bags in two marine habitats: early signs of degradation in the pelagic and benthic zone? PLoS ONE 10:e0137201. https://doi.org/10.1371/journal.pone.0137201
Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9:e111913. https://doi.org/10.1371/journal.pone.0111913
Fabra M, Williams L, Watts JE, Hale MS, Couceiro F, Preston J (2021) The plastic trojan horse: Biofilms increase microplastic uptake in marine filter feeders impacting microbial transfer and organism health. Sci Total Environ 797:149217. https://doi.org/10.1016/j.scitotenv.2021.149217
Frère L, Maignien L, Chalopin M, Huvet A, Rinnert E, Morrison H, Kerninon S, Cassone AL, Lambert C, Reveillaud J, Paul-Pont I (2018) Microplastic bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut 242:614–625. https://doi.org/10.1016/j.envpol.2018.07.023
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782. https://doi.org/10.1126/sciadv.1700782
Gkoutselis G, Rohrbach S, Harjes J, Obst M, Brachmann A, Horn MA, Rambold G (2021) Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci Rep 11:13214. https://doi.org/10.1038/s41598-021-92405-7
Gola D, Tyagi PK, Arya A, Chauhan N, Agarwal M, Singh SK, Gola S (2021) The impact of microplastics on marine environment: a review. Environ Nanotechnol Monit Manag 16:100552. https://doi.org/10.1016/j.enmm.2021.100552
Goswami P, Vinithkumar NV, Dharani G (2020) First evidence of microplastics bioaccumulation by marine organisms in the Port Blair Bay, Andaman Islands. Mar Pollut Bull 155:111163. https://doi.org/10.1016/j.marpolbul.2020.111163
Guan J, Qi K, Wang J, Wang W, Wang Z, Lu N, Qu J (2020) Microplastics as an emerging anthropogenic vector of trace metals in freshwater: significance of biofilms and comparison with natural substrates. Water Res 184:116205. https://doi.org/10.1016/j.watres.2020.116205
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9. http://palaeo-electronica.org
Harrison JP, Schratzberger M, Sapp M, Osborn AM (2014) Rapid bacterial colonisation of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol 14:1–5. https://doi.org/10.1186/s12866-014-0232-4
Junaid M, Liu S, Liao H, Liu X, Wu Y, Wang J (2022a) Wastewater Plastisphere enhances antibiotic resistant elements, bacterial pathogens, and toxicological impacts in the environment. Sci Total Environ 841:156805. https://doi.org/10.1016/j.scitotenv.2022.156805
Junaid M, Liu X, Wu Y, Wang J (2022b) Selective enrichment of antibiotic resistome and bacterial pathogens by aquatic microplastics. J Hazard Mater Adv 7:100106. https://doi.org/10.1016/j.hazadv.2022.100106
Karthik R, Robin RS, Purvaja R, Ganguly D, Anandavelu I, Raghuraman R, Ramesh R (2018) Microplastics along the beaches of southeast coast of India. Sci Total Environ 645:1388–1399. https://doi.org/10.1016/j.scitotenv.2018.07.242
Kaur K, Reddy S, Barathe P, Oak U, Shriram V, Kharat SS, Govarthanan M, Kumar V (2022) Microplastic-associated pathogens and antimicrobial resistance in environment. Chemosphere 291:133005. https://doi.org/10.1016/j.chemosphere.2021.133005
Khalid N, Aqeel M, Noman A, Khan SM, Akhter N (2021) Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ Pollut 290:118104. https://doi.org/10.1016/j.envpol.2021.118104
Khare T, Mathur V, Kumar V (2024) Agro-ecological Microplastics enriching the antibiotic resistance in aquatic environment. Curr Opin Environ Sci Health 37:100534. https://doi.org/10.1016/j.coesh.2024.100534
Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4:e01067. https://doi.org/10.1016/j.heliyon.2018.e01067
Kumar M, Kumar R, Chaudhary DR, Jha B (2022) An appraisal of early-stage biofilm-forming bacterial community assemblage and diversity in the Arabian Sea, India. Mar Pollut Bull 180:113732. https://doi.org/10.1016/j.marpolbul.2022.113732
Kumar R, Sinha R, Rakib MR, Padha S, Ivy N, Bhattacharya S, Dhar A, Sharma P (2022b) Microplastics pollution load in sundarban delta of Bay of Bengal. J Hazard Mater Adv 7:100099. https://doi.org/10.1016/j.hazadv.2022.100099
Kumkar P, Gosavi SM, Verma CR, Pise M, Kalous L (2021) Big eyes can’t see microplastics: feeding selectivity and eco-morphological adaptations in oral cavity affect microplastic uptake in mud-dwelling amphibious mudskipper fish. Sci Total Environ 786:147445. https://doi.org/10.1016/j.scitotenv.2021.147445
Kumkar P, Verma CR, Hýsek S, Pise M, Źółtowska S, Gosavi SM, Mercl F, Božik M, Praus L, Hanková K, Rinn R, Klouček P, Petrtýl M, Kalous L (2023) Contaminants and their ecological risk assessment in beach sediments and water along the Maharashtra coast of India: a comprehensive approach using microplastics, heavy metals (loids), pharmaceuticals, personal care products and plasticisers. Sci Total Environ 892:164712. https://doi.org/10.1016/j.scitotenv.2023.164712
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467. https://doi.org/10.1016/j.envpol.2018.02.050
Li W, Zhang Y, Wu N, Zhao Z, Xu W, Ma Y, Niu Z (2019) Colonisation characteristics of bacterial communities on plastic debris, influenced by environmental factors and polymer types in the Haihe Estuary of Bohai Bay, China. Environ Sci Technol 53:10763–10773. https://doi.org/10.1021/acs.est.9b03659
McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ (2014) Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol 48:11863–11871. https://doi.org/10.1021/es503610r
Metcalf R, Oliver DM, Moresco V, Quilliam RS (2022a) Quantifying the importance of plastic pollution for the dissemination of human pathogens: the challenges of choosing an appropriate ‘control’ material. Sci Total Environ 810:152292. https://doi.org/10.1016/j.scitotenv.2021.152292
Metcalf R, White HL, Moresco V, Ormsby MJ, Oliver DM, Quilliam RS (2022b) Sewage-associated plastic waste washed up on beaches can act as a reservoir for faecal bacteria, potential human pathogens, and genes for antimicrobial resistance. Mar Pollut Bull 180:113766. https://doi.org/10.1016/j.marpolbul.2022.113766
Miao L, Yu Y, Adyel TM, Wang C, Liu Z, Liu S, Huang L, You G, Meng M, Qu H, Hou J (2021) Distinct microbial metabolic activities of biofilms colonising microplastics in three freshwater ecosystems. J Hazard Mater 403:123577. https://doi.org/10.1016/j.jhazmat.2020.123577
Naidoo T, Thompson RC, Rajkaran A (2020) Quantification and characterisation of microplastics ingested by selected juvenile fish species associated with mangroves in KwaZulu Natal, South Africa. Environ Pollut 257:113635. https://doi.org/10.1016/j.envpol.2019.113635
Oberbeckmann S, Loeder MG, Gerdts MG, Osborn AM (2014) Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in northern European waters. FEMS Microb Ecol 90:478–492. https://doi.org/10.1111/1574-6941.12409
Oberbeckmann S, Löder MG, Labrenz M (2015) Marine microplastic-associated biofilms–a review. Environ Chem 12:551–562. https://doi.org/10.1071/EN15069
Ory NC, Sobral P, Ferreira JL, Thiel M (2017) Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci Total Environ 586:430–437. https://doi.org/10.1016/j.scitotenv.2017.01.175
Pan Z, Zhang C, Wang S, Sun D, Zhou A, Xie S, Xu G, Zou J (2021) Occurrence of microplastics in the gastrointestinal tract and gills of fish from Guangdong, South China. J Mar Sci Eng 9:981. https://doi.org/10.1016/j.envpol.2019.113734
Patchaiyappan A, ZakiAhmed S, Dowarah K, Khadanga SS, Singh T, Jayakumar S, Thirunavukkarasu C, Devipriya SP (2021) Prevalence of microplastics in the sediments of Odisha beaches, southeastern coast of India. Mar Pollut 167:112265. https://doi.org/10.1016/j.marpolbul.2021.112265
Perumal K, Muthuramalingam S (2022) Global sources, abundance, size, and distribution of microplastics in marine sediments-A critical review. Estuar Coast Shelf Sci 264:107702. https://doi.org/10.1016/j.ecss.2021.107702
Peters CA, Bratton SP (2016) Urbanisation is a major influence on microplastic ingestion by sunfish in the Brazos River Basin, Central Texas, USA. Environ Pollut 210:380–387. https://doi.org/10.1016/j.envpol.2016.01.018
Priyadarshanee M, Das S (2023) Bacterial extracellular polymeric substances: biosynthesis and interaction with environmental pollutants. Chemosphere 332:138876. https://doi.org/10.1016/j.chemosphere.2023.138876
Robin RS, Karthik R, Purvaja R, Ganguly D, Anandavelu I, Mugilarasan M, Ramesh R (2020) Holistic assessment of microplastics in various coastal environmental matrices, southwest coast of India. Sci Total Environ 703:134947. https://doi.org/10.1016/j.scitotenv.2019.134947
Roman L, Schuyler Q, Wilcox C, Hardesty BD (2021) Plastic pollution is killing marine megafauna, but how do we prioritise policies to reduce mortality? Conserv Lett 14:e12781. https://doi.org/10.1111/conl.12781
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4:258–267. https://doi.org/10.1021/acs.estlett.7b00164
Sambandam M, Dhineka K, Sivadas SK, Kaviarasan T, Begum M, Hoehn D, Sivyer D, Mishra P, Murthy MR (2022) Occurrence, characterisation, and source delineation of microplastics in the coastal waters and shelf sediments of the central east coast of India. Bay Bengal Chemosphere 303:135135. https://doi.org/10.1016/j.chemosphere.2022.135135
Sánchez C (2020) Fungal potential for the degradation of petroleum-based polymers: an overview of macro- and microplastics biodegradation. Biotechnol Adv 40:107501. https://doi.org/10.1016/j.biotechadv.2019.107501
Shruti VC, Jonathan MP, Rodriguez-Espinosa PF, Rodríguez-González F (2019) Microplastics in freshwater sediments of Atoyac River basin, Puebla city, Mexico. Sci Total Environ 654:154–163. https://doi.org/10.1016/j.scitotenv.2018.11.054
Sooriyakumar P, Bolan N, Kumar M, Singh L, Yu Y, Li Y, Weralupitiya C, Vithanage M, Ramanayaka S, Sarkar B, Wang F (2022) Biofilm formation and its implications on the properties and fate of microplastics in aquatic environments: a review. J Hazard Mater Adv 6:100077. https://doi.org/10.1016/j.hazadv.2022.100077
Stabnikova O, Stabnikov V, Marinin A, Klavins M, Klavins L, Vaseashta A (2021) Microbial life on the surface of microplastics in natural waters. Appl Sci 11:11692. https://doi.org/10.3390/app112411692
Stenger KS, Wikmark OG, Bezuidenhout CC, Molale-Tom LG (2021) Microplastics pollution in the ocean: potential carrier of resistant bacteria and resistance genes. Environ Pollut 291:118130. https://doi.org/10.1016/j.envpol.2021.118130
Tanaka K, Takada H (2016) Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci Rep 6:34351. https://doi.org/10.1038/srep34351
Thompson RC, Olsen Y, Mitchell P, Davis A, Rowland SJ, John AW, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838. https://doi.org/10.1126/science.1094559
Tiwari M, Rathod TD, Ajmal PY, Bhangare RC, Sahu SK (2019) Distribution and characterisation of microplastics in beach sand from three different Indian coastal environments. Mar Pollut Bull 140:262–273. https://doi.org/10.1016/j.marpolbul.2019.01.055
Tu C, Chen T, Zhou Q, Liu Y, Wei J, Waniek JJ, Luo Y (2020) Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci Total Environ 734:139237. https://doi.org/10.1016/j.scitotenv.2020.139237
Turner A, Holmes L (2011) Occurrence, distribution and characteristics of beached plastic production pellets on the island of Malta (central Mediterranean). Mar Pollut Bull 62:377–381. https://doi.org/10.1016/j.marpolbul.2010.09.027
Vaseashta A, Ivanov V, Stabnikov V, Marinin A (2021) Environmental safety and security investigations of neustonic microplastic aggregates near water-air interphase. Pol J Environ Stud 30:3457–3469. https://doi.org/10.15244/pjoes/131947
Veerasingam S, Saha M, Suneel V, Vethamony P, Rodrigues AC, Bhattacharyya S, Naik BG (2016) Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa Coast, India. Chemosphere 159:496–505. https://doi.org/10.1016/j.chemosphere.2016.06.056
Verdú I, Amariei G, Rueda-Varela C, González-Pleiter M, Leganés F, Rosal R, Fernández-Piñas F (2023) Biofilm formation strongly influences the vector transport of triclosan-loaded polyethylene microplastics. Sci Total Environ 859:160231. https://doi.org/10.1016/j.scitotenv.2022.160231
Verma CR, Pise M, Kumkar P, Gosavi SM, Kalous L (2022) Microplastic Contamination in Ulhas River Flowing through India’s most populous Metropolitan Area. Water Air Soil Pollut 233:520. https://doi.org/10.1007/s11270-022-05968-0
Wang X, Bolan N, Tsang DC, Sarkar B, Bradney L, Li Y (2021a) A review of microplastics aggregation in aquatic environment: influence factors, analytical methods, and environmental implications. J Hazard Mater 402:123496. https://doi.org/10.1016/j.jhazmat.2020.123496
Wang L, Tong J, Li Y, Zhu J, Zhang W, Niu L, Zhang H (2021b) Bacterial and fungal assemblages and functions associated with biofilms differ between diverse types of plastic debris in a freshwater system. Environ Res 196:110371. https://doi.org/10.1016/j.envres.2020.110371
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GL, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC (2014) The deep sea is a major sink for microplastic debris. Roy Soc Open Sci 1:140317. https://doi.org/10.1098/rsos.140317
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the plastisphere: microbial communities on plastic marine debris. Environ Sci Technol 47:7137–7146. https://doi.org/10.1021/es401288x
Zhang B, Yang X, Liu L, Chen L, Teng J, Zhu X, Zhao J, Wang Q (2021) Spatial and seasonal variations in biofilm formation on microplastics in coastal waters. Sci Total Environ 770:145303. https://doi.org/10.1016/j.scitotenv.2021.145303
Acknowledgements
The authors are grateful to the anonymous reviewers for offering constructive feedback on the previous version of the manuscript, which greatly improved its quality.
Funding
Open access publishing supported by the National Technical Library in Prague. The study was partially funded by IRP MSMT60460709 (PK, CV, MP, LK) and the grant of the Czech University of Life Sciences Prague No. SV23-11-21260.
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
CRV: Conceptualization, Writing- Original draft preparation, Methodology. MP: Methodology, Software. ŠH: Methodology, Visualization.SŹ: Methodology. PK: Conceptualization, Visualization, Formal analysis. LK: Conceptualisation, Supervision, Resources. SMG: Conceptualization, Formal analysis, Visualisation, Writing- Original draft preparation.All authors contributed to Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verma, C.R., Pise, M., Hýsek, Š. et al. Occurrence and Distribution of Plastispheres in Coastal Sediments and Waters along the Maharashtra Coast, India. Thalassas (2024). https://doi.org/10.1007/s41208-024-00710-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41208-024-00710-5