Abstract
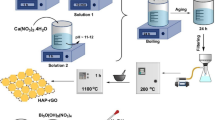
The study of the physicochemical properties of water and the removal of water contaminants using an active adsorbent material is a vital approach today. The purpose of this study is to modify orange peel (OP) using polyaniline (PANI) via in situ oxidative polymerization method and characterize it for wastewater treatment applications. The properties of water were analyzed according to the standard guideline published by APH before the adsorption study. The proposed materials were characterized by SEM, FTIR, and UV–VIS spectroscopy. The modification of orange peel using polyaniline was confirmed by electronic transition and stretching vibration peaks obtained from FT-IR and UV–Vis spectroscopies and its morphologies from SEM. The results of most of the physicochemical properties, nutrients, and heavy metals were above the acceptable range for wastewater discharge limits set by FAO, WHO, and EEPA. The Cu and Zn adsorption performance of as-synthesized materials was studied and depicted a high adsorption capacity for copper (176.9 mg/g) and zinc (151.3 mg/g) in wastewater solutions. When all parameters were optimized (pH at 6, contact time at 40 min, temperature at 300 K, and 1 gr of PANI-OP), 90.03% removal of copper and 85% (pH at 4, contact time at 60 min, temperature at 300 K, and 1 gr of PANI-OP) removal of zinc were observed. The adsorption equilibriums of both copper and zinc were best described by the Freundlich isotherm model. Therefore, the synthesized novel material PANI-OP is a promising candidate for the removal of Cu and Zn from wastewater.












Similar content being viewed by others
Data availability
Materials are available for all.
Code availability
Not applicable.
References
Lin L, Yang H, Xu X (2022) Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front Environ Sci 10 https://doi.org/10.3389/fenvs.2022.880246
Hama Aziz, K.H., et al.: Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC Adv. 13, 17595–17610 (2023). https://doi.org/10.1039/d3ra00723e
Rajasulochana, P., Preethy, V.: Comparison on efficiency of various techniques in treatment of waste and sewage water – A comprehensive review. Resour Technol 2, 175–184 (2016). https://doi.org/10.1016/j.reffit.2016.09.004
Ali, I., Gupta, V.K.: Advances in water treatment by adsorption technology. Nat. Protoc. 1, 2661–2667 (2007). https://doi.org/10.1038/nprot.2006.370
Bhatnagar, A., Sillanpää, M.: Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem Eng J 157, 277–296 (2010). https://doi.org/10.1016/j.cej.2010.01.007
Lee, B.G., Rowell, R.M.: Removal of heavy metal ions from aqueous solutions using lignocellulosic fibers. J Nat Fibers 1, 97–108 (2004). https://doi.org/10.1300/J395v01n01_07
Michael-Igolima, U., Abbey, S.J., Ifelebuegu, A.O., Eyo, E.U.: Modified Orange Peel Waste as a Sustainable Material for Adsorption of Contaminants. Mater 16, 1092 (2023). https://doi.org/10.3390/ma16031092
Liu L et al. (2021) Adsorption Performance of La(III) and Y(III) on Orange Peel: Impact of Experimental Variables, Isotherms, and Kinetics. Adsorpt Sci Technol 2021 https://doi.org/10.1155/2021/7189639
Hasan, M.B., Al-Tameemi, I.M., Abbas, M.N.: Orange Peels as a Sustainable Material for Treating Water Polluted with Antimony. J Ecol Eng 22, 25–35 (2021). https://doi.org/10.12911/22998993/130632
Pan, B., Pan, B., Zhang, W., Lv, L., Zhang, Q., Zheng, S.: Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J 151, 19–29 (2009). https://doi.org/10.1016/j.cej.2009.02.036
Sajid, M., Nazal, M.K., Baig, N., Osman, A.M.: Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: A critical review. Sep Purif Technol 191, 400–423 (2018). https://doi.org/10.1016/j.seppur.2017.09.011
Taghizadeh, A., et al.: Conductive polymers in water treatment: A review. J. Mol. Liq. 312, 113447 (2020). https://doi.org/10.1016/j.molliq.2020.113447
Khashij, M., Moheb, A., Mehralian, M., Gharloghi, M.: Modeling of the adsorption breakthrough behaviors of 4-chlorophenol in a fixed bed of nano graphene oxide adsorbent. J Water Supply Res Technol 65, 2–8 (2016)
Tan, I.A.W., Ahmad, A.L., Hameed, B.H.: Fixed-bed adsorption performance of oil palm shell-based activated carbon for removal of 2,4,6-trichlorophenol. Bioresour. Technol. 100, 1494–1496 (2009)
Teklu T et al. (2020a) Polyaniline Deposition on the Surface of Cotton Fibers : Structural Studies , Swelling Behavior , and Water Absorption Properties. 2020
Teklu T et al. (2020b) Polyaniline Deposition on the Surface of Cotton Fibers: Structural Studies, Swelling Behavior, and Water Absorption Properties. Adv Mater Sci Eng 2020 https://doi.org/10.1155/2020/1650364
Teklu, T., Wangatia, L.M., Alemayehu, E.: In situ deposition of polyaniline onto sisal fibers and removal efficiency for chromium ( VI ) from aqueous solution: Structure and adsorption studies. J Appl Surfaces Interfaces 3, 1–9 (2018). https://doi.org/10.48442/IMIST.PRSM/jasi-v3i1-3.11304
Khalil, A., Salem, M., Ragab, S., Sillanpää, M., El Nemr, A.: Orange peels magnetic activate carbon (MG-OPAC) composite formation for toxic chromium absorption from wastewater. Sci. Rep. 13, 1–17 (2023). https://doi.org/10.1038/s41598-023-30161-6
Jadoun, S., Fuentes, J.P., Urbano, B.F., Yáñez, J.: A review on adsorption of heavy metals from wastewater using conducting polymer-based materials. J. Environ. Chem. Eng. 11, 109212–109226 (2023). https://doi.org/10.1016/j.jece.2022.109226
Samadi, A., Xie, M., Li, J., Shon, H., Zheng, C., Zhao, S.: Polyaniline-based adsorbents for aqueous pollutants removal: A review. Chem Eng J 418, 129413–129425 (2021). https://doi.org/10.1016/j.cej.2021.129425
Buonomenna, M.G., Mousavi, S.M., Hashemi, S.A., Lai, C.W.: Water Cleaning Adsorptive Membranes for Efficient Removal of Heavy Metals and Metalloids. Water 14, 2710–2718 (2022). https://doi.org/10.3390/w14172718
Shimizu, S., Matubayasi, N.: Understanding sorption mechanism directly from Isotherms. Langumer 39, 6113–6125 (2023). https://doi.org/10.1021/acs.langumuir.3c00256
Kalaichelvi, K., Dhivya, S.M.: Screening of phytoconstituents, UV-VIS Spectrum and FTIR analysis of Micrococca mercurialis (L) Benth. Int J Herb Med 5, 40–44 (2017)
Jonas Wolfs, F., Scheelje, C.M., Matveyeva, O., Meier, M.A.R.: Determination of the degree of substitution of cellulose esters via ATR-FTIR spectroscopy. J. Polym. Sci. 6, 152–165 (2023). https://doi.org/10.1002/pol.20230220
Oh, S.Y., Yoo, D.I., Shin, Y., Seo, G.: FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr Res 340, 417–428 (2005). https://doi.org/10.1016/j.carres.2004.11.027
Ozuomba, J.O.: Synthesis and Characterization of Acid-Doped Polyaniline Thin Films. Niger. J. Technol. 37, 135–138 (2018). https://doi.org/10.4314/njt.v37i1.18
Israel, L.L., et al.: Insights into the Electrochemical Behavior and Kinetics of NiP@PANI/ rGO as a High-Performance Electrode for Alkaline Urea Oxidation. Electrocatalysis 13, 1–16 (2022). https://doi.org/10.1007/s12678-022-00718-6
Akinhanmi, T.F., Ofudje, E.A., Adeogun, A.I., Aina, P., Joseph, I.M.: Orange peel as low - cost adsorbent in the elimination of Cd ( II ) ion : kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour Bioprocess (2020). https://doi.org/10.1186/s40643-020-00320-y
Mafra, M.R., Igarashi-Mafra, L., Zuim, D.R., Vasques, É.C., Ferreira, M.A.: Adsorption of remazol brilliant blue on an orange peel adsorbent. Brazilian J Chem Eng 30, 657–665 (2013). https://doi.org/10.1590/S0104-66322013000300022
Ambalagi, S.M., Devendrappa, M., Nagaraja, S., Sannakki, B.: Dielectric Properties of PANI/CuO Nanocomposites. IOP Conf Ser Mater Sci Eng 310, 012081 (2018). https://doi.org/10.1088/1757-899X/310/1/012081
Razalli, R.L., et al.: Polyaniline-modified nanocellulose prepared from Semantan bamboo by chemical polymerization: Preparation and characterization. RSC Adv. 7, 25191–25198 (2017). https://doi.org/10.1039/c7ra03379f
Charity Eboagu, N., Ishmael EgbulefuAjiwe, V., ChukwuemekaAralu, C., EkwyOchiagha, K., Joy Morah, E.: Assessment of Physicochemical Parameters of Water from Selected Boreholes Around Nnewi Industrial Area, Anambra State, Nigeria. Am J Environ Sci Eng 7, 23–33 (2023). https://doi.org/10.11648/j.ajese.20230701.14
Protection TE, United T, Industrial N (2003) AMBIENT ENVIRONMENT STANDARDS FOR environmental protection.
Khuhawar, M.Y., Mirza, M.A., Leghari, S.M., Arain, R.: Limnological study of baghsar lake district. Pak. J. Bot. 41, 1903–1915 (2009)
Lianthuamluaia, Landge A. T., Purushothamen CS, Deshmukha G. RKK (2013) Assessment of Seasonal Variations of Water Quality Parameters of Savitri Reservoir, Poladpur, Raigad District, Maharashtra. Krish 8:1337–1342. https://krishi.icar.gov.in/jspui/handle/123456789/53284
Fitriani, N., Wahyudianto, F.E., Salsabila, N.F., Mohamed, R.M., Kurniawan, S.B.: Performance of modified slow sand filter to reduce turbidity, total suspended solids and iron in river water as water treatment i disaster areas. J Ecol Eng 24, 1–18 (2023). https://doi.org/10.12911/22998993/156009
Moklesur Rahman, M., Haque, T., Azhar Mahmud, M.A.A.: Drinking water quality assessment based on index values incorporating WHO guidelines and Bangladesh standards. Phys. Chem. Earth Parts A/B/C. 129, 103353 (2023). https://doi.org/10.1016/j.pce.2022.103353
Guo, X., Shuzhen Zhang, X.S.: Adsorption of metal ions on lignin. J. Hazard. Mater. 151, 134–142 (2008). https://doi.org/10.1016/j.jhazmat.2007.05.065
Jiang, N., Yiting, Xu., Dai, Y., Weiang Luo, L.D.: Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. J. Hazard. Mater. 215, 17–24 (2012). https://doi.org/10.1016/j.jhazmat.2012.02.026
Ghorbani, M., Eisazadeh, H., Ghoreyshi, A.A., Engineering, C., Box, P.O.: Removal of Zinc ions from aqueous solution using polyaniline nanocomposite coated on rice husk. Iran. J. Energy Environ. 3(1), 66–71 (2012). https://doi.org/10.5829/idosi.ijee.2012.03.01.3343
Kanwal, F., Batool, A., Rasool, S., Naseem, S., Rehman, R.: Synthesis of polyaniline composites with grinded leaves of Polyalthia longifolia, Syzygium cumini, Alstonia scholaris and Madhuca longifolia and study of their structural, electrical and dielectric properties. Asian J. Chem. 26, 7519–7522 (2014). https://doi.org/10.14233/ajchem.2014.16649
Kanwal, F., Rehman, R., Samson, S., Anwar, J.: Isothermal investigation of Copper(II) and Nickel(II) adsorption from water by novel synthesized polyaniline composites with Polyalthia longifolia and Alastonia scholaris dried leaves. Asian J. Chem. 25, 9013–9019 (2013). https://doi.org/10.14233/ajchem.2013.14968
Karthikeyan, G., Andal, N.M.: Adsorption studies of iron(III) on chitin. J Chem Sci 117, 663–672 (2005). https://doi.org/10.1007/BF02708296
Özacar, M., Şengil, İA.: Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour Technol 96, 791–795 (2005). https://doi.org/10.1016/j.biortech.2004.07.011
Padmavathy, K.S., Madhu, G., Haseena, P.V.: A study on Effects of pH, Adsorbent Dosage, Time, Initial Concentration and Adsorption Isotherm Study for the Removal of Hexavalent Chromium (Cr (VI)) from Wastewater by Magnetite Nanoparticles. Procedia Technol 24, 585–594 (2016). https://doi.org/10.1016/j.protcy.2016.05.127
Chen, X., Hossain, M.F., Duan, C., Lu, J., Tsang, Y.F., Islam, M.S., Zhou, Y.: Isotherm models for adsorption of heavy metals from water - A review. Chemosphere 307, 135535–135545 (2022). https://doi.org/10.1016/j.chemosphere.2022.135545
Mohammad, A., Al-Ghouti, D.A.D.: Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 393, 122374–122383 (2020). https://doi.org/10.1016/j.jhazmat.2020.122383
Kanwal F, Rehman R, Anwar J, Saeed M (2012) Batchwise Removal of Chromium ( VI ) by Adsorption on Novel Synthesized Polyaniline Composites with Various Brans and Isothermal Modeling of Equilibrium Data. JChemSocPak 34:1134–1139. https://www.researchgate.net/232746313
Khan, M.A., Otero, M., Kazi, M., Alqadami, A.A., Wabaidur, S.M., Siddiqui, M.R., Alothman, Z.A., Sumbul, S.: Unary and binary adsorption studies of lead and malachite green onto a nanomagnetic copper ferrite/drumstick pod biomass composite. J. Hazard. Mater. 365, 759–770 (2019)
Teklu, T., Wangatia, L.M., Alemayehu, E.: Removal of Pb(II) from aqueous media using adsorption onto polyaniline coated sisal fibers. J. Vinyl. Addit. Technol. 25, 189–197 (2019). https://doi.org/10.1002/vnl.21652
Gupta, V.K., Ganjali, M.R., Nayak, A., Bhushan, B., Agarwal, S.: Enhanced heavy metals removal and recovery by mesoporous adsorbent prepared from waste rubber tire. Chem. Eng. J. 197, 330–342 (2012). https://doi.org/10.1016/j.cej.2012.04.104
Funding
We are grateful for supporting this research work with fund by Wolkite University, Ethiopia. All the laboratory activity were carried out at Wolkite University, Zebidar brewery S.C and Addis Ababa University, Arat kilo campus, Ethiopia.
Author information
Authors and Affiliations
Contributions
Israel Leka: conceptualization, methodology, investigation, writing-original draft, writing-review and editing, validation, project administration Sutripto Khanshi: Resources, validation, editing, facilitating Lodrick Wangatia: data curation, supervision, project administration, Femi Olu: data curation, supervision Praveen C. Ramamurthy: Supervision, validation, Resources, material funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
No ethics problem.
Consent to participate
Participate at all processes.
Consent for publication
Participate at all processes.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diro, S.T., Bahru, T.B. & Lera, I.L. Polyaniline doping induced abundant active sites in orange peel as an efficient adsorbent material for water treatment. Adsorption (2024). https://doi.org/10.1007/s10450-024-00466-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00466-7