Abstract
Dihydrogen bond (DHB) interaction that exists in ammoniated metal borohydride systems is recognized as an effective intermediate step involved in the evolution of H2 molecules. Mechanism of DHB formation and its electronic properties upon halogenations were explored for Mg(BH4)2·2NH3⋯M(BH3X) (where M = Li, Na, K, and X = H, F, Cl, and Br) systems using ab initio (MP2) and DFT (ωB97XD) calculations. The influence of halogens in varying the nature of the DHB that forms in Mg(BH4)2·2NH3⋯M(BH3X) complexes was explored with the Quantum Theory of Atoms In Molecule (QTAIM) analysis. Further, Energy decomposition analysis (EDA) was made to understand the strength of DHB interaction through the calculation of the Edisp term in interaction energy. The results obtained from EDA and QTAIM were found to correlate well with the structural parameter and the interaction energy values. This study reveals the influence of halogenations on tuning the electronic properties of DHB interaction in all the complexes. Our results suggest that the effective substitution of halogens in BH4 molecule enhances DHB interaction, and the impact of halogenations on DHB has been revealed through QTAIM, EDA, Natural Bond Order (NBO), Non-covalent Interaction (NCI), and Bader charge analyses.
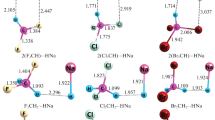
Graphical abstract
-
Dihydrogen bonds in ammine metal borohydride systems are responsible for its large hydrogen storage capacity.
-
This study reveals the DHB interaction found in the chosen Mg(BH4)2.2NH3⋯M(BH3X) systems and their property enhancement upon introducing halogens through QTAIM parameters.




Similar content being viewed by others
References
Comanescu C 2022 Complex metal borohydrides: From laboratory oddities to prime candidates in energy storage applications Materials 15 2286
Schneemann A, White J L, Kang S, Jeong S, Wan L F, Cho E S, et al. 2018 Nanostructured metal hydrides for hydrogen storage Chem. Rev. 118 10775
Wang K, Pan Z and Yu X 2019 Metal B-N-H hydrogen-storage compound: Development and perspectives J. Alloys. Compd. 794 303
Kumar R, Karkamkar A, Bowden M and Autrey T 2019 Solid-state hydrogen rich boron-nitrogen compounds for energy storage Chem. Soc. Rev. 48 5350
Ouyang L, Chen K, Jiang J, Yang X S and Zhu M 2020 Hydrogen storage in light-metal based systems: A review J. Alloys. Compd. 829 154597
Richardson T B, de Gala S, Crabtree R H M and Siegbahn P E 1995 Unconventional Hydrogen Bonds: Intermolecular B-H-*H-N Interactions C. E. Acta Crystallogr. Soc. 117
Soloveichik G, Her J H, Stephens P W, Gao Y, Rijssenbeek J, Andrus M and Zhao J C 2008 Ammine magnesium borohydride complex as a new material for hydrogen storage: Structure and properties of Mg(BH4)2· 2NH3 Inorg. Chem. 47 4290
Custelcean R and Jackson J E 2001 Dihydrogen bonding: Structures, energetics, and dynamics Chem. Rev. 101 1963
Belkova N V, Epstein L M, Filippov O A and Shubina E S 2016 Hydrogen and dihydrogen bonds in the reactions of metal hydrides Chem. Rev. 116 8545
Makepeace J W, He T, Weidenthaler C, Jensen T R, Chang F, Vegge T, et al. 2019 Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress Int. J. Hydrog. Energy 44 7746
Chen X, Yuan F, Gu Q, Tan Y, Liu H, Dou S, et al. 2013 Improved dehydrogenation properties of the combined Mg(BH4) 2·6NH3-nNH3BH3 system Int. J. Hydrog. Energy 38 16199
Yan Y, Grinderslev J B, Lee Y S, Jørgensen M, Cho Y W, Černý R and Jensen T R 2020 Ammonia-assisted fast Li-ion conductivity in a new hemiammine lithium borohydride, LiBH4·1/2NH3 Chem. Commun. 56 3971
Ulucan T H, Akhade S A, Ambalakatte A, Autrey T, Cairns A, Chen P, Cho Y W, Gallucci F Gao W and Grinderslev J B 2023 Hydrogen storage in liquid hydrogen carriers: recent activities and new trends Prog. Energy 5
Peng B and Chen J 2008 Ammonia borane as an efficient and lightweight hydrogen storage medium Energy Environ Sci. 1 479
Chen X, Zhao J C and Shore S G 2013 The roles of dihydrogen bonds in amine borane chemistry Acc. Chem. Res. 46 2666
Chu H, Qiu S, Sun L and Xu F 2015 Advanced Materials for Renewable Hydrogen Production, Storage and Utilization Chapter 3 53 (INTECH)
Jepsen L H, Ley M B, Filinchuk Y, Besenbacher F and Jensen T R 2015 Tailoring the properties of ammine metal borohydrides for solid-state hydrogen storage ChemSusChem. 8 1452
Zheng X, Wu G, Li W, Xiong Z, He T, Guo J, Chen H and Chen P 2011 Releasing 17.8 wt% H 2 from lithium borohydride ammoniate Energy Environ. Sci. 4 3593
Guo Y, Jiang Y, Xia G and Yu X 2012 Ammine aluminium borohydrides: An appealing system releasing over 12 wt% pure H2 under moderate temperature Chem. Commun. 48 4408
Grinderslev J B, Amdisen M B and Jensen T R 2020 Synthesis, crystal structures and thermal properties of ammine barium borohydrides Inorganics (Basel). 8 1
Paskevicius M, Jepsen L H, Schouwink P, Černý R, Ravnsbæk D B, Filinchuk Y, et al. 2017 Metal borohydrides and derivatives-synthesis, structure and properties Chem. Soc. Rev. 46 1565
Filippov S, Grinderslev J B, Andersson M S, Armstrong J, Karlsson M, Jensen T R, et al. 2019 Analysis of dihydrogen bonding in ammonium borohydride J. Phys. Chem. C 123 28631
Milanese C, Jensen T R, Hauback B C, Pistidda C, Dornheim M, Yang H, et al. 2019 Complex hydrides for energy storage Int. J. Hydrog. Energy 44 7860
Dematteis E M, Amdisen M B, Autrey T, Barale J, Bowden M E, Buckley C E, Cho Y W, Deledda S, Dornheim M, Jongh P D, Grinderslev J B, Gizer G, Gulino V, Hauback B C, Heere M, Heo T W, Humphries T D, Jensen T R, Kang S Y, Lee Y S, Li H W, Li S, Møller K T, Ngene P, Orimo S, Paskevicius M, Polanski M, Takagi S, Wan L, Wood B C, Hirscher M and Baricco M 2022 Hydrogen storage in complex hydrides: Past activities and new trends Prog. Energy 4
Hirscher M, Yartys V A, Baricco M, Bellosta von Colbe J, Blanchard D, Bowman R C, et al. 2020 Materials for hydrogen-based energy storage – past, recent progress and future outlook J. Alloys Compd. 827 153548
Sun W, Chen X, Gu Q, Wallwork K S, Tan Y, Tang Z and Yu X 2012 A new ammine dual-cation (Li, Mg) borohydride: Synthesis, structure, and dehydrogenation enhancement Chem. Eur. J. 18 6825
Yang Y, Liu Y, Li Y, Gao M and Pan H 2013 Heating rate-dependent dehydrogenation in the thermal decomposition process of Mg(BH4)2·6NH3 J. Phys. Chem. C 117 16326
Guo Y, Wu H, Zhou W and Yu X 2011 Dehydrogenation tuning of ammine borohydrides using double-metal cations J. Am. Chem. Soc. 133 4690
Yang Y, Liu Y, Li Y, Gao M and Pan H 2013 Synthesis and thermal decomposition behaviors of magnesium borohydride ammoniates with controllable composition as hydrogen storage materials Chem. Asian J. 8 476
Yang Y, Liu Y, Wu H, Zhou W, Gao M and Pan H 2014 An ammonia-stabilized mixed-cation borohydride: Synthesis, structure and thermal decomposition behavior Phys. Chem. Chem. Phys. 16 135
Arumugam S, Angamuthu A and Gopalan P 2021 Molecular insights on the dihydrogen bond properties of metal borohydride complexes upon ammoniation ECS J. Solid State Sci. Technol. 10 091006
Welchman E and Thonhauser T 2017 Decomposition mechanisms in metal borohydrides and their ammoniates J. Mater. Chem. A 5 4084
Nakamori Y, Miwa K, Ninomiya A, Li H, Ohba N, Towata S I, et al. 2006 Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments Phys. Rev. B 74 1
Arnbjerg L M, Ravnsbæk D B, Filinchuk Y, Vang R T, Cerenius Y, Besenbacher F, et al. 2009 Structure and dynamics for LiBH4 - LiCl solid solutions Chem. Mater. 21 5772
Golub I E, Filippov O A, Gulyaeva E S, Gutsul EI and Belkova N V 2017 The interplay of proton accepting and hydride donor abilities in the mechanism of step-wise boron hydrides alcoholysis Inorg. Chim. Acta 456 113
Golub I E, Filippov O A, Belkova N V, Epstein L M and Shubina E S 2021 The reaction of hydrogen halides with tetrahydroborate anion and hexahydro-closo-hexaborate dianion Molecules 26
Yang Y, Liu Y, Li Y, Gao M and Pan H 2015 Fluorine-substituted Mg(BH4)2·2NH3 with improved dehydrogenation properties for hydrogen storage J. Mater. Chem. A 3 570
Sharma M, Sethio D, D’Anna V and Hagemann H 2015 Theoretical study of B 2Hn F (12 - N) 2 -species Int. J. Hydrog. Energy 40 12721
Head-Gordon M and Head-Gordon T 1994 Analytic MP2 frequencies without fifth-order storage. Theory and application to bifurcated hydrogen bonds in the water hexamer Chem. Phys. Lett. 220 122
Chai J, Da and Head-Gordon M 2008 Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections Phys. Chem. Chem. Phys. 10 6615
Zhou Y, Yoshida K, Yamaguchi T, Liu H, Fang C and Fang Y 2017 Microhydration of BH4-: Dihydrogen bonds, structure, stability, and Raman spectra J. Phys. Chem. A 121 9146
Chemcraft - Citation [Internet]. [cited 2023 Mar 23]. Available from: https://www.chemcraftprog.com/citation.html
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E Jr, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Keith T, Kobayashi R, Normand J, Raghavachari K, Kendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas O, Foresman J B, Ortiz J V, Cioslowski J and Fox D J (2009) Gaussian 09, revision A.1. Gaussian Inc, Wallingford
Jamróz M H 2013 Vibrational energy distribution analysis (VEDA): Scopes and limitations Spectrochim. Acta A 114 220
Lu T and Chen F 2012 Multiwfn: A multifunctional wavefunction analyzer J. Comput. Chem. 33 580
Johnson E R, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen A J and Yang W 2010 Revealing noncovalent interactions J. Am. Chem. Soc. 132 6498
Reed A E, Curtiss L A and Weinhold F 1988 Intermolecular interactions from a natural bond orbital, donor—Acceptor viewpoint Chem. Rev. 88 899
Boys S F and Bernardi F 1970 The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors Mol. Phys. 19 553
Meot-Ner M 2005 The ionic hydrogen bond Chem. Rev. 105 213
Pegu D, Deb J, Van Alsenoy C and Sarkar U 2017 Theoretical investigation of electronic, vibrational, and nonlinear optical properties of 4-fluoro-4-hydroxybenzophenone Spectro. Lett. 4 232
Darugar V, Vakili M, Tayyari S F and Kamounah F S 2021 Validation of potential energy distribution by VEDA in vibrational assignment some of β-diketones; comparison of theoretical predictions and experimental vibration shifts upon deutration J. Mol Graph Model 1 107
Gunasekaran S, Kumaresan S, Arun Balaji R, Anand G and Seshadri S 2008 Vibrational spectra and normal coordinate analysis on structure of chlorambucil and thioguanine Pram. - J. Phys. 6 1291
Jash P, Meux K and Trenary M 2012 Transmission infrared spectroscopy of ammonia borane J. Undergrad. Res. 5 1
Kumar P S V, Raghavendra V and Subramanian V 2016 Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding J. Chem. Sci. 128 1527
Espinosa E, Molins E and Lecomte C 1998 Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities Chem. Phys. Lett. 285 170
Popelier P L A 1998 Characterization of a dihydrogen bond on the basis of the electron density J. Phys. Chem. A 102 1873
Vijaya Pandiyan B, Deepa P and Kolandaivel P 2015 Do resonance-assisted intramolecular halogen bonds exist without a charge transfer and a σ-hole? Phys. Chem. Chem. Phys. 17 27496
Duraisamy P D, Gopalan P and Angamuthu A 2020 Intermolecular C-H⋯H-M dihydrogen bonds in five-membered heterocyclic complexes: a DFT and ab-initio study Monatsh. Chem. 151 1569
Golub I E, Filippov O A, Gutsul E I, Belkova N V, Epstein L M, Rossin A, et al. 2012 Dimerization mechanism of bis(triphenylphosphine)copper(I) tetrahydroborate: Proton transfer via a dihydrogen bond Inorg. Chem. 51 6486
Golub I E, Filippov O A, Belkova N V, Epstein L M, Rossin A, Peruzzini M and Shubina E S 2016 Two pathways of proton transfer reaction to (triphos)Cu(η1-BH4): Via a dihydrogen bond [triphos = 1,1,1-tris(diphenyl phosphinomethyl)ethane] Dalton Trans. 45 9127
Paul D, Deb J and Sarkar U 2020 A detailed DFT study on electronic structures and nonlinear optical properties of doped C30 Chem. Select 23 6987
Paul D, Dua H and Sarkar U 2020 Confinement effects of a noble gas dimer inside a fullerene cage: Can it be used as an acceptor in a DSSC? Front. Chem. 6 8
Yan Y, Dononelli W, Jørgensen M, Grinderslev J B, Lee Y S, Cho Y W, et al. 2020 The mechanism of Mg2+ conduction in ammine magnesium borohydride promoted by a neutral molecule Phys. Chem. Chem. Phys. 22 9204
Contreras-García J, Yang W and Johnson E R 2011 Analysis of hydrogen-bond interaction potentials from the electron density: Integration of noncovalent interaction regions J. Phys. Chem. A 115 12983
Contreras-garcía J, Johnson E R, Keinan S, Chaudret R, Piquemal P, Beratan D N and Yang W 2012 NCIPLOT: a program for plotting non-covalent interaction regions J. Chem. Theory Comput. 7 625
Sagan F, Filas R and Mitoraj M P 2016 Non-covalent interactions in hydrogen storage materials LiN(CH3)2BH3 and KN(CH3)2BH3 Crystals (Basel) 6 11
Arunan E, Desiraju G R, Klein R A, Sadlej J, Scheiner S, Alkorta I, et al. 2011 Definition of the hydrogen bond (IUPAC Recommendations 2011) Pure Appl. Chem. 83 1637
Golub I E, Gulyaeva E S, Filippov O A, Dyadchenko V P, Belkova N V, Epstein L M, et al. 2015 Dihydrogen bond intermediated alcoholysis of dimethylamine-borane in nonaqueous media J. Phys. Chem. A 119 3853
Parida R, Inostroza Rivera R, Sinha S and Giri S 2021 Can superhalogen ligand make more reactive frustrated Lewis Pairs? Chem. Select 6 8068
Freindorf M, Mccutcheon M, Beiranvand N and Kraka E 2023 Dihydrogen bonding- seen through the eyes of vibrational spectroscopy Molecules 28 263
Bandyopadhyay P, Karmakar A, Deb J, Sarkar U and Seikh M M 2020 Non-covalent interactions between epinephrine and nitroaromatic compounds: A DFT study Spectrochim. Acta A 5 228
Deb J, Paul D, Sarkar U and Ayers P W 2018 Characterizing the sensitivity of bonds to the curvature of carbon nanotubes J. Mol. Model. 9 24
Pawar R and Subramanian V 2019 Hydrogen bonding interaction of N5H with water: A first principle calculations Comput. Theor. Chem. 1165 1
Author information
Authors and Affiliations
Contributions
Saravanapriya Arumugam: Methodology, Formal analysis, Validation, Visualization. Abiram Angamuthu: Methodology, Validation and Visualization. Praveena Gopalan: Supervision, Project administration, Writing – review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arumugam, S., Angamuthu, A. & Gopalan, P. Effect of boron halogenation on dihydrogen bonds: A quantum mechanical approach. J Chem Sci 136, 28 (2024). https://doi.org/10.1007/s12039-024-02258-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-024-02258-6