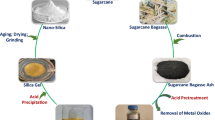
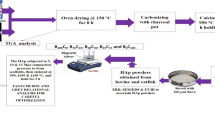
Abstract
Copper ions are prevalent in the natural environment and possess toxicity as heavy metal ions. The removal of Cu2+ from aqueous solutions can be achieved through adsorption, which is considered a straightforward method. In this study, we employed a facile approach to synthesize pig-bone hydroxyapatite material (pHAP), and the synthesized materials were subjected to characterization using XRD, SEM, FTIR, and BET techniques. The results showed that pHAP has a pure HAP structure, and the surface of HAP has a certain porosity, which provides good conditions for the adsorption of copper ions. Batch adsorption equilibrium experiments were conducted to investigate the various influencing factors, adsorption kinetics, and isotherms. The findings revealed that the optimal adsorption condition of Cu2+ (50 mg/L) on pHAP was pH 7, 318.15 K, the maximum adsorption capacity was 50.25 mg/g, and the adsorption capacity was superior to some adsorbents of the same type. Moreover, it can retain 74.15% of its reusability after being reused 5 times. The adsorption mechanism primarily involves monolayer adsorption through chemical processes, particularly ion exchange, coprecipitation, and complexation reactions. Therefore, pHAP has industrial application potential in the field of copper ion adsorption.









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Parmar, M., Thakur, L.S.: Heavy metal Cu, Ni and Zn: toxicity, health hazards and their removal techniques by low cost adsorbents: a short overview. Int. J. Plant Anim. Environ. Sci. 3(3), 143–157 (2013)
Xia, Y.-D., Sun, Y.-Q., Cheng, Y., et al.: Rational design of dual-ligand Eu-MOF for ratiometric fluorescence sensing Cu2+ ions in human serum to diagnose Wilson’s disease. Anal. Chim. Acta 1204, 339731 (2022)
Liu, M., Zhu, H., Wang, K., et al.: A novel fluorescent chemodosimeter for discriminative detection of Cu2+ in environmental, food, and biological samples. ACS Food Sci. Technol. 3(1), 134–140 (2022)
Zhong, W., Zou, J., Yu, Q., et al.: Ultrasensitive indirect electrochemical sensing of thiabendazole in fruit and water by the anodic stripping voltammetry of Cu2+ with hierarchical Ti3C2Tx-TiO2 for signal amplification. Food Chem. 402, 134379 (2023)
Bijimol, D., Punnoose, M.S., Korah, B.K., et al.: Fluorescent sensor based on thiourea capped Mn doped ZnS quantum dots for the sensing of Cu2+ ions in water. Environ. Nanotechnol. Monit. Manag. 18, 100710 (2022)
Lin, Y.-W., Peng, S.-Y., Lee, W.-H., et al.: Characterization of Cu2+ adsorption for eco-hydroxyapatite derived from limestone sludge via hydrothermal synthesis. J. Mater. Cycles Waste Manage. 25(2), 1069–1081 (2023)
Kim, H.Y., Song, J., Park, H.G., et al.: Electrochemical detection of zeptomolar miRNA using an RNA-triggered Cu2+ reduction method. Sens. Actuators, B Chem. 360, 131666 (2022)
Gao, D.L., Lin, W.W., Lin, Q.J., et al.: Remarkable adsorption capacity of Cu2+-doped ZnAl layered double hydroxides and the calcined materials toward phosphate. J. Environ. Chem. Eng. 11(2), 109472 (2023)
Xiong, Q., Zhang, F.: Study on the performance of composite adsorption of Cu2+ by Chitosan/β-Cyclodextrin cross-linked zeolite. Sustainability 14(4), 2106 (2022)
Cao, X., Meng, Z., Song, E., et al.: Co-adsorption capabilities and mechanisms of bentonite enhanced sludge biochar for de-risking norfloxacin and Cu2+ contaminated water. Chemosphere 299, 134414 (2022)
Sellaoui, L., Dhaouadi, F., Taamalli, S., et al.: Understanding the Cu2+ adsorption mechanism on activated carbon using advanced statistical physics modelling. Environ. Sci. Pollut. Res. 29(36), 54882–54889 (2022)
Khalif, M., Daneshmehr, S., Arshadi, S., et al.: Adsorption of O2 molecule on the transition metals (TM (II)= Sc2+, Ti2+, V2+, Cr2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+ and Zn2+) porphyrins induced carbon nanocone (TM (II) PCNC). J. Mol. Graph. Model. 119, 108362 (2023)
Zhou, Y., Jiang, B., Wang, Y., et al.: Effect of Cu2+ on the laccase-inducted formation of non-extractable residues of tetrabromobisphenol a in humic acids. Bull. Environ. Contam. Toxicol. 109(6), 1162–1166 (2022)
Bao, Y., Jin, J., Ma, M., et al.: Ion exchange conversion of Na-Birnessite to Mg-Buserite for enhanced and preferential Cu2+ removal via hybrid capacitive deionization. ACS Appl. Mater. Interfaces. 14(41), 46646–46656 (2022)
Arokiasamy, P., Abdullah, M.M.A.B., Abd Rahim, S.Z., et al.: Synthesis methods of hydroxyapatite from natural sources: A review. Ceram. Int. 48(11), 14959–14979 (2022)
Balasooriya, I.L., Chen, J., KoraleGedara, S.M., et al.: Applications of nano hydroxyapatite as adsorbents: A review. Nanomaterials 12(14), 2324 (2022)
DileepKumar, V.G., Sridhar, M.S., Aramwit, P., et al.: A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 33(2), 229–261 (2022)
Amenaghawon, A.N., Anyalewechi, C.L., Darmokoesoemo, H., et al.: Hydroxyapatite-based adsorbents: Applications in sequestering heavy metals and dyes. J. Environ. Manage. 302, 113989 (2022)
Zheng, Y., Zhang, J.: Experimental study on the adsorption of dissolved heavy metals by nano-hydroxyapatite. Water Sci. Technol. 82(9), 1825–1832 (2020)
Dabiri, S.M.H., Rezaie, A.A., Moghimi, M., et al.: Extraction of hydroxyapatite from fish bones and its application in nickel adsorption. BioNanoScience 8(3), 823–834 (2018). https://doi.org/10.1007/s12668-018-0547-y
Zhou, Y., Chang, D., Chang, J.: Preparation of nano-structured pig bone hydroxyapatite for high-efficiency adsorption of Pb2+ from aqueous solution. Int. J. Appl. Ceram. Technol. 14(6), 1125–1133 (2017). https://doi.org/10.1111/ijac.12749
Wetlesen, C.U.: Rapid spectrophotometric determination of copper in iron, steel and ferrous alloys. Anal. Chim. Acta. 16, 268–270 (1957)
Derderian, M.D.: Determination of calcium and magnesium in plant material with EDTA. Anal. Chem. 33(12), 1796–1798 (1961)
Lagergren, S.K.: About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar. 24, 1–39 (1898)
Ho, Y.-S., McKay, G.: Sorption of dye from aqueous solution by peat. Chem. Eng. J. 70(2), 115–124 (1998)
Ho, Y.S., McKay, G.: Pseudo-second order model for sorption processes. Process Biochem. 34(5), 451–465 (1999). https://doi.org/10.1016/S0032-9592(98)00112-5
Ho, Y.S., McKay, G.: The sorption of lead(II) ions on peat. Water Res. 33(2), 578–584 (1999). https://doi.org/10.1016/S0043-1354(98)00207-3
Pan, Y., Li, Z., Zhang, Z., et al.: Adsorptive removal of phenol from aqueous solution with zeolitic imidazolate framework-67. J. Environ. Manage. 169, 167–173 (2016). https://doi.org/10.1016/j.jenvman.2015.12.030
Freundlich, H.: Over the adsorption in solution. J. Phys. Chem. 57(385471), 1100–1107 (1906)
Langmuir, I.: The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 38(11), 2221–2295 (1916)
Mathias, P.M.: The Gibbs-Helmholtz equation in chemical process technology. Ind. Eng. Chem. Res. 55(4), 1076–1087 (2016)
Ayodele, O., Olusegun, S.J., Oluwasina, O.O., et al.: Experimental and theoretical studies of the adsorption of Cu and Ni ions from wastewater by hydroxyapatite derived from eggshells. Environ. Nanotechnol. Monit. Manag. 15, 100439 (2021). https://doi.org/10.1016/j.enmm.2021.100439
Dong, Q., Chen, Z., Zhao, B., et al.: In situ fabrication of niobium pentoxide/graphitic carbon nitride type-II heterojunctions for enhanced photocatalytic hydrogen evolution reaction. J. Colloid Interface Sci. 608, 1951–1959 (2022). https://doi.org/10.1016/j.jcis.2021.10.161
Zhu, G., Jin, Y., Ge, M.: Simple and green method for preparing copper nanoparticles supported on carbonized cotton as a heterogeneous Fenton-like catalyst. Colloids Surf., A 647, 128978 (2022). https://doi.org/10.1016/j.colsurfa.2022.128978
Zhang, L., Lu, T., He, F., et al.: Physicochemical and cytological properties of poorly crystalline calcium-deficient hydroxyapatite with different Ca/P ratios. Ceram. Int. 48(17), 24765–24776 (2022). https://doi.org/10.1016/j.ceramint.2022.05.126
Lakrat, M., Jodati, H., Mejdoubi, E.M., et al.: Synthesis and characterization of pure and Mg, Cu, Ag, and Sr doped calcium-deficient hydroxyapatite from brushite as precursor using the dissolution-precipitation method. Powder Technol. 413, 118026 (2023). https://doi.org/10.1016/j.powtec.2022.118026
Uskoković, V., Odsinada, R., Djordjevic, S., et al.: Dynamic light scattering and zeta potential of colloidal mixtures of amelogenin and hydroxyapatite in calcium and phosphate rich ionic milieus. Arch. Oral Biol. 56(6), 521–532 (2011). https://doi.org/10.1016/j.archoralbio.2010.11.011
Kong, L., Liu, X., Lv, G., et al.: Copper adsorption using hydroxyapatite derived from bovine bone. Adv. Civ. Eng. 2022 (2022)
Yang, W., Cao, M.: Study on the difference in adsorption performance of graphene oxide and carboxylated graphene oxide for Cu(II), Pb(II) respectively and mechanism analysis. Diam. Relat. Mater. 129, 109332 (2022). https://doi.org/10.1016/j.diamond.2022.109332
Akhyar, H.M., Djuned, F., Mulyati, S., et al.: Effectivity of dolomite adsorbent in purification of Mn and Cu from acid mine drainage. Int. J. S. Sci. Educ. Econ. Agric. Res. Technol. 2(6), 86–96 (2023)
Yulianti, F., Kurniawati, D., Oktavia, B., et al.: Immobilization of langsat shell (lansium domesticum) to absorption of metal ions Cu. In: AIP Publishing. 2673(1), 060009 (2023)
Xinyu, Z., Tao, F., Hongwei, Y., et al.: Preparation of chitosan-like magnetic composite microspheres and their adsorption properties for Cu (II). Mater. Today: Proc. 71, 105–113 (2022). https://doi.org/10.1016/j.matpr.2022.09.616
Jung, K.-W., Lee, S.Y., Choi, J.-W., et al.: A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media. Chem. Eng. J. 369, 529–541 (2019). https://doi.org/10.1016/j.cej.2019.03.102
Joshi, P., Manocha, S.: Kinetic and thermodynamic studies of the adsorption of copper ions on hydroxyapatite nanoparticles. Mater. Today: Proc. 4(9), 10455–10459 (2017). https://doi.org/10.1016/j.matpr.2017.06.399
Le, H.R., Chen, K.Y., Wang, C.A.: Effect of pH and temperature on the morphology and phases of co-precipitated hydroxyapatite. J. Sol-Gel. Sci. Technol. 61(3), 592–599 (2012)
Shao, F., Xu, J., Chen, F., et al.: Insights into olation reaction-driven coagulation and adsorption: A pathway for exploiting the surface properties of biochar. Sci. Total Environ. 854, 158595 (2023). https://doi.org/10.1016/j.scitotenv.2022.158595
Funding
This work was supported by the Excellent Discipline Cultivation Project by JHUN (2023XKZ043).
Author information
Authors and Affiliations
Contributions
Ling Shi: Conceptualization, Writing–original draft, Writing–review & editing. Zhongkui Zhu: Data curation, Formal Analysis, Investigation, Methodology. Nana Wu: Project administration, Resources, Investigation. Yufeng Chang: Project administration, Resources, Investigation. Lin Yue: Investigation, Methodology. Liang An: Investigation, Methodology.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was not required for this research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, L., Zhu, Z., Wu, N. et al. Natural hydroxyapatite powder from pig-bone waste (pHAP) for the rapid adsorption of heavy metals (Cu) in aqueous solution. Adsorption (2024). https://doi.org/10.1007/s10450-024-00471-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00471-w