Abstract
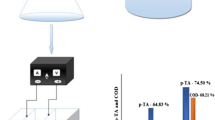
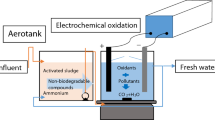
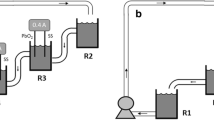
In this study, EC process using an aluminum anode, and EC-EO process using aluminum and mixed metal oxide, i.e., platinum-ruthenium dioxide-coated onto titanium (Al-Ti/Pt-RuO2) anode was used to understand the remove of phenolic syntan (PS) from synthetic tannery wastewaters. The operational conditions of the abovementioned electrochemical processes were optimized using Taguchi L16 method in terms of maximum removal of total organic carbon (TOC) and PS. At the optimum operating condition (current density = 14.25 mA/cm2, initial pH = 4, rotational speed = 70 rpm and initial PS amount = 0.25 g/L), the incomplete removal of TOC (83.93%) and PS (81.19%) was obtained in the EC process with the energy consumption of 0.135 kWh/g TOC remove and 0.056 kWh/g PS remove. In contrast, almost (≈100%) complete removal of the dissolved organic pollutant was observed in the EC-EO process with the energy consumption of 0.113 kWh/g TOC remove and 0.0453 kWh/g PS remove. The energy consumption per g TOC and PS removed was 0.135 and 0.056 kWh for the EC process, whereas 0.113 and 0.0453 kWh for the EC-EO process. The operating cost of the EC-EO process was estimated to be 1.39 USD/m3, which was lesser (-19.65%) than the operating cost of the EC process. Signal-to-noise ratio and ANOVA results showed that current density was the most influential parameter with the highest delta value and contribution ratio for TOC and PS removal in both the EC and EC-EO process. The UV/Vis and FT-IR analyses indicate that the highest removal of aromatic compounds was obtained in the EC-EO process compared to the EC process. FT-IR analyses confirmed that the PS was first degraded into a quinone functional group, which was further oxidized into carboxylic acid.











Similar content being viewed by others
Data Availability
The collection of data in a recent study are based on experiments performed by the author for his PhD program and can be shared on reasonable request with due permission of his thesis supervisor and the institute.
References
Aber, S., Amani-Ghadim, A. R., & Mirzajani, V. (2006). Removal of Cr(VI) from polluted solutions by electrocoagulation: Modeling of experimental results using artificial neural network. Journal of Hazardous Materials, 171, 484–490.
Adhoum, N., & Monser, L. (2004). Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chemical Engineering and Processing: Process Intensification, 43(10), 1281–1287. https://doi.org/10.1016/j.cep.2003.12.00110.1016/j.cep.2003.12.001
Afroze, S., & Sen, T. K. (2018). A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water, Air, & Soil Pollution, 229(7). https://doi.org/10.1007/s11270-018-3869-z https://doi.org/10.1007/s11270-018-3869-z
AlJaberi, F. Y. (2019). Modelling current efficiency and ohmic potential drop in an innovated electrocoagulation reactor. Desalination and Water Treatment, 164, 102–110. https://doi.org/10.5004/dwt.2019.24452
Anglada, Á., Urtiaga, A. M., & Ortiz, I. (2010). Laboratory and pilot plant scale study on the electrochemical oxidation of landfill leachate. Journal of Hazardous Materials, 181(1–3), 729–735. https://doi.org/10.1016/j.jhazmat.2010.05.07310.1016/j.jhazmat.2010.05.073
Balki, M. K., Sayin, C., & Sarıkaya, M. (2016). Optimization of the operating parameters based on Taguchi method in an SI engine used pure gasoline, ethanol and methanol. Fuel, 180, 630–637. https://doi.org/10.1016/j.fuel.2016.04.098
Belaid, C., Khadraoui, M., Mseddi, S., Kallel, M., Elleuch, B., & Fauvarque, J. F. (2013). Electrochemical treatment of olive mill wastewater: Treatment extent and effluent phenolic compounds monitoring using some uncommon analytical tools. Journal of Environmental Sciences, 25(1), 220–230. https://doi.org/10.1016/s1001-0742(12)60037-0
Benhadji, A., Ahmed, M. T., & Maachi, R. (2011). Electrocoagulation and effect of cathode materials on the removal of pollutants from tannery wastewater of Rouïba. Desalination, 277, 128–134.
Buso, A., Balbo, L., Giomo, M., Farnia, G., & Sandonà, G. (2000). Electrochemical Removal of Tannins from Aqueous Solutions. Industrial & Engineering Chemistry Research, 39(2), 494–499. https://doi.org/10.1021/ie990192a.59830.10.1039/c4ra09069a
Can, O. T., Kobya, M., Demirbas, E., & Bayramoglu, M. (2006). Treatment of the textile wastewater by combined electrocoagulation. Chemosphere, 62(2), 181–187.
Cañizares, P., García-Gómez, J., Sáez, C., & Rodrigo, M. A. (2004). Electrochemical oxidation of several chlorophenols on diamond electrodes: Part II. Influence of waste characteristics and operating conditions. Journal of Applied Electrochemistry, 34, 87–94. https://doi.org/10.1023/B:JACH.0000005587.52946.66
Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38, 11–41. https://doi.org/10.1016/j.seppur.2003.10.006
Ciorba, G., Radovan, C., Vlaicu, I., & Masu, S. (2002). Removal of nonylphenol ethoxylates by electrochemically-generated coagulants. Journal of Applied Electrochemistry, 32, 561–567. https://doi.org/10.1023/A:1016577230769
Daghrir, R., Drogui, P., Blais, J. F., & Mercier, G. (2012). Hybrid Process Combining Electrocoagulation and Electro-Oxidation Processes for the Treatment of Restaurant Wastewaters. Journal of Environmental Engineering, 138, 1146–1156.
Daneshvar, N., Oladegaragoze, A., & Djafarzadeh, N. (2006). Decolorization of basic dye solutions by electrocoagulation: An investigation of the effect of operational parameters. Journal of Hazardous Materials, 129(1–3), 116–122.
Danhong, S., Qiuang, H., Wenjun, Z., Yulu, W., & Bi, S. (2008). Evaluation of environmental impact of typical leather chemicals. Part II, biodegradability of organic tanning agents by activated sludge. Journal of the Society of Leather Technologists and Chemists, 92(2), 59–64.
Dhawane, S. H., Kumar, T., & Halder, G. (2016). Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: Optimization by Taguchi method. Renewable Energy, 89, 506–514.
Dirany, A., Sirés, I., Oturan, N., Özcan, A., & Oturan, M. A. (2012). Electrochemical Treatment of the Antibiotic Sulfachloropyridazine: Kinetics, Reaction Pathways, and Toxicity Evolution. Environmental Science & Technology, 46(7), 4074–4082. https://doi.org/10.1021/es204621q10.1021/es204621q
El-Ashtoukhy, E.-S.Z., Amin, N. K., & Abdelwahab, O. (2009). Treatment of paper mill effluents in a batch-stirred electrochemical tank reactor. Chemical Engineering Journal, 146(2), 205–210. https://doi.org/10.1016/j.cej.2008.05.037
Fajardo, A. S., Seca, H. F., Martins, R. C., Corceiro, V. N., Freitas, I. F., Quinta-Ferreira, M. E., & Quinta-Ferreira, R. M. (2017). Electrochemical oxidation of phenolic wastewaters using a batch-stirred reactor with NaCl electrolyte and Ti/RuO2 anodes. Journal of Electroanalytical Chemistry, 785, 180–189. https://doi.org/10.1016/j.jelechem.2016.12.03310.1016/j.jelechem.2016.12.033
Feng, Y. J., & Li, X. Y. (2003). Electro-catalytic oxidation of phenol on several metal-oxide electrodes in aqueous solution. Water Research, 37(10), 2399–2407. https://doi.org/10.1016/s0043-1354(03)00026-510.1016/s0043-1354(03)00026-5
Ferella, F., Michelis, L. D., Zerbini, C., & Veglio, F. (2013). Advanced treatment of industrial wastewater by membrane filtration and ozonisation. Desalination, 313, 1–11.
Fernandes, A., Santos, D., Pacheco, M. J., Ciríaco, L., & Lopes, A. (2016). Electrochemical oxidation of humic acid and sanitary landfill leachate: Influence of anode material, chloride concentration and current density. Science of the Total Environment, 541, 282–291. https://doi.org/10.1016/j.scitotenv.2015.09.05210.1016/j.scitotenv.2015.09.052
Ganesh, R., & Ramanujam, R. A. (2009). Biological waste management of leather tannery effluentsin India: Current optionsand future research needs. International Journal of Environmental Engineering, 1, 165–186.
Garcia, S. N., Clubbs, R. L., Stanley, J. K., Scheffe, B., Yelderman, J. C., & Brooks, B. W. (2013). Comparative analysis of effluent water quality from a municipal treatment plant and two on-site wastewater treatment systems. Chemosphere, 92, 38–44. https://doi.org/10.1016/j.chemosphere.2013.03.007
Garg, K. K., & Prasad, B. (2015). Removal of para-toluic acid (p-TA) from purified terephthalic acid (PTA) waste water by electrocoagulation process. Journal of Environmental Chemical Engineering, 3(3), 1731–1739.
Geng, R., Zhao, G. H., Liu, M. C., & Lei, Y. Z. (2010). In situ ESR Study of Hydroxyl Radical Generation on a Boron Doped Diamond Film Electrode Surface. Acta Physico-Chimica Sinica, 26, 1493–1498.
Ghalwa, N. A., Tamos, H., ElAskalni, M., & El Agha, A. R. (2012). Generation of sodium hypochlorite (NaOCl) from sodium chloride solution using C/PbO2 and Pb/PbO2 electrodes. International Journal of Minerals, Metallurgy, and Materials, 19(6), 561–566. https://doi.org/10.1007/s12613-012-0596-010.1007/s12613-012-0596-0
Ginos, A., Manios, T., & Mantzavinos, D. (2006). Treatment of olive mill effluents by coagulation–flocculation hydrogen peroxide oxidation and effect on phytotoxicity. Journal of Hazardous Materials, 133, 135–142.
Gokkus, O., Yıldız, Y. S., & Yavuz, B. (2012). Optimization of chemical coagulation of real textile wastewater using Taguchi experimental design method. Desalination and Water Treatment, 49, 263–271.
Gonder, Z. B., Kaya, Y., Vergili, I., & Barlas, H. (2010). Optimization of filtration conditions for CIP wastewater treatment by nanofiltration process using Taguchi approach. Separation and Purification Technology, 70, 265–273. https://doi.org/10.1016/j.seppur.2009.10.001
Gotsi, M., Kalogerakis, N., Psillakis, E., Samaras, P., & Mantzavinos, D. (2005). Electrochemical oxidation of olive oil mill wastewaters. Water Research, 39(17), 4177–4187. https://doi.org/10.1016/j.watres.2005.07.037
Govindaraj, M., Muthukumar, M., & Bhaskar Raju, G. (2010). Electrochemical oxidation of tannic acid contaminated wastewater by RuO2/IrO2/TaO2-coated titanium and graphite anodes. Environmental Technology, 31(14), 1613–1622. https://doi.org/10.1080/09593330.2010.48214710(1080/09593330),pp.482147,2010
Gunes, S., Manay, E., Senyigit, E., & Ozceyhan, V. (2011). A Taguchi approach for optimization of design parameters in a tube with coiled wire inserts. Applied Thermal Engineering, 31, 2568–2577. https://doi.org/10.1016/j.applthermaleng.2011.04.022
Guo, Z. R., Zhang, G., Fang, J., & Dou, X. (2006). Enhanced chromium recovery from tanning wastewater. Journal of Cleaner Production, 14(1), 75–79. https://doi.org/10.1016/j.jclepro.2005.01.00510.1016/j.jclepro.2005.01.005
Gurses, A., Yalcin, M., & Dogar, C. (2022). Electrocoagulation of some reactive dyes: A statistical investigation of some electrochemical variables. Waste Management, 22, 491–499.
Hassoune, J., Tahiri, S., Aarfane, A., El krati, M., Salhi, A., & Azzi, M. (2017). Removal of Hydrolyzable and Condensed Tannins from Aqueous Solutions by Electrocoagulation Process. Journal of Environmental Engineering, 143(6), 04017010. https://doi.org/10.1061/(asce)ee.1943-7870.0001196
Heidmann, I., & Calmano, V. (2008). Removal of Zn (II), Cu(II), Ni(II), Ag(I) and Cr (VI) present in aqueous solutions by aluminium electrocoagulation. Journal of Hazardous Materials, 152(3), 934–941.
Hine, F. (1985). Electrode Processes and Electrochemical Engineering. Plenum Press.
Holt, P. H., Barton, G. W., Wark, M., & Mitchell, A. A. (2002). A quantitative comparison between chemical dosing and electrocoagulation. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 211, 233–248.
Irdemez, S., Demircioglu, N., Yıldız, Y. S., & Bingul, Z. (2006). The effects of current density and phosphate concentration on phosphate removal from wastewater by electrocoagulation using aluminum and iron plate electrodes. Separation and Purification Technology, 52, 218–223. https://doi.org/10.1016/j.seppur.2006.04.008
Jadhav, S. B., Chougule, A. S., Shah, D. P., Pereira, C. S., & Jadhav, J. P. (2014). Application of response surface methodology for the optimization of textile effluent biodecolorization and its toxicity perspectives using plant toxicity, plasmid nicking assays. Clean Technologies and Environmental Policy, 1–12. https://doi.org/10.1007/s10098-014-0827-3
Jin, P., Chang, R., Liu, D., Zhao, K., Zhang, L., & Ouyang, Y. (2014). Phenol degradation in an electrochemical system with TiO2 /activated carbon fiber as electrode. Journal of Environmental Chemical Engineering, 2(2), 1040–1047.
Karthikeyan, S., Kumar, M. A., Maharaja, P., Partheeban, T., Sridevi, J., & Sekaran, G. (2014). Process optimization for the treatment of pharmaceutical wastewater catalyzed by poly sulpha sponge. Journal of the Taiwan Institute of Chemical Engineers, 45, 1739–1747. https://doi.org/10.1016/j.jtice.2014.01.009
Khorshidi, B., Thundat, T., Fleck, B. A., & Sadrzadeh, M. (2015). Thin film composite polyamide membranes: Parametric study on the influence of synthesis conditions. RSC Advance, 5, 54985–54997. https://doi.org/10.1039/C5RA08317F
Khosla, N. K., Venkatachalam, S., & Somasundaran, P. (1991). Pulsed electro- generation of bubbles for electroflotation. Journal of Applied Electrochemistry, 21, 986–990.
Kim, T. H., Park, Ch., Shin, E. B., & Kim, S. (2002). Decolorization of disperse and reactive dyes by continuous electrocoagulation process. Desalination, 150, 165–175.
Kobya, M., Can, O. T., & Bayramoglu, M. (2003). Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. Journal of Hazardous Materials, 100(1–3), 163–178.
Kumar, A., & Basu, D. (2022a). Optimization of removal of Cr (VI) from wastewater by electrocoagulation process using Response Surface Methodology. Journal of Hazardous, Toxic, and Radioactive Waste. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000723
Kumar, A., & Basu, D. (2022b). Economic and performance evaluation of electrocoagulation unit for the treatment of hexavalent chromium using Taguchi method. International Journal of Environmental Science and Technology. https://doi.org/10.1007/s13762-022-04439-7
Kumar, A., & Basu, D. (2023). Parametric optimization of hexavalent chromium removal by electrocoagulation technology with vertical rotating cylindrical aluminum electrodes using Taguchi and ANN model. Journal of Environmental Health Science and Engineering, 21(1), 255–275.
Li, M., Feng, C., Hu, W., Zhang, Z., & Sugiura, N. (2009). Electrochemical degradation of phenol using electrodes of Ti/RuO2–Pt and Ti/IrO2–Pt. Journal of Hazardous Materials, 162(1), 455–462. https://doi.org/10.1016/j.jhazmat.2008.05.063
Li, H., Huang, G., An, C., Hu, J., & Yang, S. (2013). Removal of Tannin from Aqueous Solution by Adsorption onto Treated Coal Fly Ash: Kinetic, Equilibrium, and Thermodynamic Studies. Industrial & Engineering Chemistry Research, 52, 15923–15931.
Li, P., Cai, W., Xiao, Y., Wang, Y., & Fan, J. (2017). Electrochemical degradation of phenol wastewater by SnSb-Ce modified granular activated carbon. International Journal of Electrochemical Science, 12, 2777–2790.
Linares-Hernandez, I., Barrera-Díaz, C., Bilyeu, B., Juarez-GarcíaRojas, P., & Campos-Medina, E. (2010). A combined electrocoagulation-electrooxidation treatment for industrial wastewater. Journal of Hazardous Materials, 175, 688–694. https://doi.org/10.1016/j.jhazmat.2009.10.064
Lofrano, G., Meriç, S., Belgiorno, V., Nikolaou, A. N., Gallo, M., & Napoli, R. M. A. (2007). Fenton and Photo-Fenton treatment of a synthetic tannin used in leather tannery: A multi-approach study. Water Science and Technology, 55(10), 53–61.
Lofrano, G., Aydin, E., Russo, F., Guida, M., Belgiorno, V., & Meric, S. (2008). Characterization, fluxes and toxicity of leather tanning bath chemicals in a large tanning district area (IT). Water Air Soil Pollution, 8, 529–542. https://doi.org/10.1007/s11267-008-9177-7
Luu, T. L. (2020). Tannery wastewater treatment after activated sludge pre‑treatment using electro‑oxidation on inactive anodes. Clean Technologies and Environmental Policy. 2020. https://doi.org/10.1007/s10098-020-01907-x
Mandal, P., Dubey, B. K., & Gupta, A. K. (2017). Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Management, 69, 250–273. https://doi.org/10.1016/j.wasman.2017.08.03410.1016/j.wasman.2017.08.034
Mansouri, K., Ibrik, K., Bensalah, N., & Abdel-Wahab, A. (2011). Anodic Dissolution of Pure Aluminum during Electrocoagulation Process: Influence of Supporting Electrolyte, Initial pH, and Current Density. Industrial & Engineering Chemistry Research, 50(23), 13362–13372. https://doi.org/10.1021/ie201206d10.1021/ie201206d
Michalowicz, J., & Duda, W. (2007). Phenols – Sources and Toxicity. Polish Journal of Environmental Studies, 16(3), 347–362.
Min, K. S., Yu, J. J., Kim, Y. J., & Yun, Z. (2004). Removal of Ammonium from Tannery Wastewater by Electrochemical Treatment. Journal of Environmental Science and Health, Part A, 39(7), 1867–1879. https://doi.org/10.1081/ese-120037884
Miriam, G., Rodriguez, R., Mendoza, V., Puebla, H., Sergio, A., & Martínez, D. (2019). Removal of Cr(VI) from wastewaters at semi-industrial electrochemical reactors with rotating ring electrodes. Journal of Hazardous Materials, 163, 1221–1229.
Moreira, F. C., Boaventura, R. A. R., Brillas, E., & Vilar, V. J. P. (2017). Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Applied Catalysis b: Environmental, 202, 217–261. https://doi.org/10.1016/j.apcatb.2016.08.037
Murugananthan, M., Bhaskar Raju, G., & Prabhakar, S. (2005). Removal of tannins and polyhydroxy phenols by electrochemical techniques. Journal of Chemical Technology & Biotechnology, 80(10), 1188–1197. https://doi.org/10.1002/jctb.1314
Nicolla, E. D., Meric, S., Gallo, M., laccarino, M., Rocca, C. D., Lofrano, G., Russo, T., & Pagano, G. (2007). Vegetable and synthetic tannins induce hormesis/toxicity in sea urchin early development and in algal growth. Environmental Pollution, 146(1), 46–54.
Olya, M. E., & Pirkarami, A. (2013). Electrocoagulation for the removal of phenol and aldehyde contaminants from resin effluent. Water Science and Technology, 68(9), 1940–1949. https://doi.org/10.2166/wst.2013.439
Ozgunay, H., Colak, S., Mutlu, M. M., & Akyuz, F. (2007). Characterization of leather industry wastes. Polish Journal of Environmental Studies, 16, 867–873.
Ozyonar, F., & Korkmaz, M. U. (2022). Sequential use of the electrocoagulation-electrooxidation processes for domestic wastewater treatment. Chemosphere, 290, 133172. https://doi.org/10.1016/j.chemosphere.2021.133172
Panizza, M. (2000). Electrochemical treatment of wastewater containing polyaromatic organic pollutants. Water Research, 34(9), 2601–2605. https://doi.org/10.1016/s0043-1354(00)00145-7
Panizza, M., & Cerisola, G. (2005). Application of diamond electrodes to electrochemical processes. Electrochimca Acta, 51(2), 191–199. https://doi.org/10.1016/j.electacta.2005.04.023
Radjenovic, J., & Sedlak, D. L. (2015). Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environmental Science and Technology, 49, 11292–11302. https://doi.org/10.1021/acs.est.5b02414
Rajkumar, D., Song, B. J., & Kim, J. G. (2007). Electrochemical degradation of reactive blue 19 in chloride medium for the treatment of textile dyeing wastewater with identification of intermediate compounds. Dyes and Pigments, 72(1), 1–7.
Raju, G. B., Karuppiah, M. T., Latha, S. S., Parvathy, S., & Prabhakar, S. (2008). Treatment of wastewater from synthetic textile industry by electrocoagulation–electrooxidation. Chemical Engineering Journal, 144(1), 51–58. https://doi.org/10.1016/j.cej.2008.01.008
Rema, T., Parivallal, B., & Ramanujam, R. A. (2010). Studies on degradation of syntan used in leather tanning process using ozone. International Journal of Environmental Science and Development, 1(3), 264–267.
Rivera-Utrilla, J., Sánchez-Polo, M., & Zaror, C. A. (2002). Degradation of Naphthalene sulfonic acid by oxidation with ozone in aqueous phase. Physical Chemistry Chemical Physics, 4, 1129–1134.
Sarkka, H., Bhatnagar, A., & Sillanpaa, M. (2015). Recent developments of electrooxidation in water treatment - a review. Journal of Electroanalytical Chemistry, 754, 46–56.
Song, Z. Y., Zhou, J. T., Wang, J., Yan, B., & Du, C. H. (2003). Decolorization of azo dyes by Rhodobacter sphaeroides. Biotechnology Letters, 25, 1815–1818.
Stone, R. A., & Veevers, A. (1994). The Taguchi influence on designed experiments. Journal of Chemometrics, 8(2), 103–110.
Sun, D., Hong, X., Wu, K., Hui, K. S., Du, Y., & Hui, K. N. (2020). Simultaneous removal of ammonia and phosphate by electro-oxidation and electrocoagulation using RuO2-IrO2/Ti and microscale zero-valent iron composite electrode. Water Research, 169, 115239. https://doi.org/10.1016/j.watres.2019.115239
Sundarapandiyan, S., Chandrasekar, R., Ramanaiah, B., Krishnan, S., & Saravanan, P. (2010). Electrochemical oxidation and reuse of tannery saline wastewater. Journal of Hazardous Materials, 180(1–3), 197–203. https://doi.org/10.1016/j.jhazmat.2010.04.013
Sundarapandiyan, S., Renitha, T. S., Sridevi, J., Chandrasekaran, B., Saravanan, P., & Raju, G. B. (2014). Mechanistic insight into active chlorine species mediated electrochemical degradation of recalcitrant phenolic polymers. RSC Advances, 4(104), 59821–59830. https://doi.org/10.1039/c4ra09069a
Sundarapandiyan, S., Renitha, T. S., Sridevi, J., Saravanan, P., Chandrasekaran, B., & Raju, G. B. (2017). Photocatalytic degradation of highly refractive phenolic polymer – Mechanistic insights as revealed by Electron Spin Resonance (ESR) and solid-state 13C NMR spectroscopy. Chemical Engineering Journal, 313, 1112–1121. https://doi.org/10.1016/j.cej.2016.11.009
Taguchi, G. (1990). Introduction to Quality Engineering. McGraw-Hill.
Taguchi, G. (1986). Introduction to quality engineering: designing quality into products and processes. Asian Productivity Organization, Tokyo.
Tavares, M. G., da Silva, L. V. A., Sales Solano, A. M., Tonholo, J., Martínez-Huitle, C. A., & Zanta, C. L. P. S. (2012). Electrochemical oxidation of Methyl Red using Ti/Ru0.3Ti0.7O2 and Ti/Pt anodes. Chemical Engineering Journal, 204–206, 141–150. https://doi.org/10.1016/j.cej.2012.07.056
Thankappan, R., Srinivasan, S. V., Suthanthararajan, R., & Sillanpää, M. (2017). Studies on removal of phenol sulfonic acid-syntan in aqueous medium using ozonation. Environmental Technology. https://doi.org/10.1080/09593330.2017.1355936
Tisler, T., & Koncan, J. Z. (1997). Comparative assessment of toxicity of phenol, formaldehyde and industrial wastewater to aquatic organisms. Water, Air & Soil Pollution, 97, 315–322.
Tripathi, M., Vikram, S., Jain, R. K., & Garg, S. K. (2011). Isolation and growth characteristics of chromium (VI) and pentachlorophenol tolerantbacterial isolate from treated tannery effluent for its possible use in simultaneous bioremediation. Indian Journal of Microbiology, 51(1), 61–69.
Vijayalakshmi, P., Raju, G. B., & Gnanamani, A. (2011). Advanced Oxidation and Electrooxidation As Tertiary Treatment Techniques to Improve the Purity of Tannery Wastewater. Industrial & Engineering Chemistry Research, 50(17), 10194–10200.
Virginija, J., Jiyembetova, I., Gulbinieniene, A., Sirvaityte, J., Beleska, K., & Urbelis, V. (2014). Comparable evaluation of leather waterproofing behaviour upon hide quality II. influence of retannig and fatliquoring agents on leather structure and properties. Materials Science, 20(2), 165–170.
Vocciante, M., Menshova, I. I., & Ferro, S. (2021). Electrochemical Incineration of Synthetic Tannins Used in Retanning Processes. Theoretical Foundations of Chemical Engineering, 55(4), 618–627.
Wu, Z., & Zhou, M. (2001). Partial Degradation of Phenol by Advanced Electrochemical Oxidation Process. Environmental Science & Technology, 35(13), 2698–2703. https://doi.org/10.1021/es001652q10.1021/es001652q
Yoon, J. H., Yang, J., Shim, Y. B., & Won, M. S. (2007). Electrochemical degradation of benzoquinone in a flow through cell with carbon fiber. Bulletin of the Korean Chemical Society, 28, 403–407.
Zhu, X., Ni, J., Wei, J., Xing, X., Li, H., & Jiang, Y. (2010). Scale-up of BDD anode system for electrochemical oxidation of phenol simulated wastewater in continuous mode. Journal of Hazardous Materials, 184, 493–498. https://doi.org/10.1016/j.jhazmat.2010.08.062
Zolgharnein, J., Asanjrani, N., & Shariatmanesh, T. (2013). Taguchi L16 orthogonal array optimization for cd(II) removal using Carpinus betulus tree leaves: Adsorption characterization. International Biodeterioration & Biodegradation, 85, 66–77.
Zolgharnein, J., Asanjrani, N., Bagtash, M., & Azimi, G. (2014). Multi-response optimization using Taguchi design and principle component analysis for removing binary mixture of alizarin red and alizarin yellow from aqueous solution by nano γ-alumina. Spectrochimica Acta Part a: Molecular and Biomolecular Spectroscopy, 126, 291–300.
Acknowledgements
The authors are grateful to the Department of Civil Engineering at MNNIT Allahabad for all their assistance in carrying out the research and to colleagues for providing valuable input and help at various stages of the investigation.
Funding
NA.
Author information
Authors and Affiliations
Contributions
Every author helped to discover concepts and design. AK was in charge of designing the experimental apparatus and material preparation. Under the guidance of DB, AK carried out experimentation and data analysis. AK wrote the initial draft of the manuscript, which was subsequently improved with the help of all of the authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of Interest
The authors declare that they have no Conflict of Interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Basu, D. Comparison of electrocoagulation and combined electrocoagulation-electrooxidation treatment for synthetic tannery wastewaters bearing phenolic syntan. Water Air Soil Pollut 235, 259 (2024). https://doi.org/10.1007/s11270-024-07058-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07058-9