Abstract
Background
The use of immune checkpoint inhibitors has led to an increase in randomized controlled trials exploring various first-line combination treatment regimens. With the introduction of new PD-1/PD-L1 inhibitors, there are now more clinical options available. For the first time, the AK105 monoclonal antibody Penpulimab, developed in China, was included. The AK105-302 Phase III trial studied the efficacy and safety of Penpulimab combined with chemotherapy in patients with advanced or metastatic squamous NSCLC. To determine the optimal treatment options, we conducted an updated network meta-analysis to compare the effectiveness and safety of these regimens.
Methods
The system retrieves data from Chinese and English electronic databases, Clinical Trials, and the gov Clinical Trial Registration website up to September 6, 2023. The study indirectly compared the efficacy and safety of PD-1/PD-L1 combination regimens, including overall survival (OS), progression-free survival (PFS), objective response rate (ORR), all-grade adverse events, and above-grade III adverse events. Subgroup analyses were conducted based on programmed death ligand 1 (PD-L1) level, histological type, ECOG score, sex, and smoking history.
Results
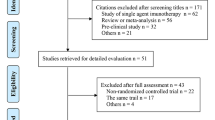
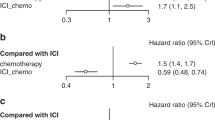
Nineteen RCTS were included, with a total of ten thousand eight hundred patients. Penpulimab plus chemotherapy (Pen + CT) provided the best OS (HR = 0.55, 95% CI 0.38–0.81) for PD-L1 patients with non-selective advanced NSCLC. Except Nivolumab plus Ipilimumab (Niv + Ipi), other PD-1/PD-L1 combination therapies significantly extended PFS compared with CT, and Nivolumab plus Bevacizumab combined with chemotherapy (Niv + Bev + CT) (HR = 0.43, 95% CI 0.26–0.74) provided the best PFS benefit and was comparable to Pen + CT (HR = 1.0) for PFS prolongation. For ORR, except Niv + Ipi, all the other regimens significantly improved ORR compared with CT. In terms of safety, except Tor + CT, the incidence of any-grade AEs or grade ≥ 3 adverse events may be higher than those of chemotherapy. The subgroup analysis revealed that for patients with PD-L1 levels below 1%, treatment with Tor + CT resulted in the best progression-free survival (HR = 0.47, 95% CI 0.25–0.86). For patients with PD-L1 levels of 1% or higher, Sintilimab plus chemotherapy (Sin + CT) (HR = 0.56, 95% CI 0.31–0.99) and Camrelizumab plus chemotherapy (Cam + CT) (HR = 0.43, 95% CI 0.28–0.64) were associated with the best overall survival and progression-free survival, respectively. For patients with SqNSCLC, combined immunotherapy may provide greater survival benefits. For patients with Non-sqNSCLC, Niv + Bev + CT and Tor + CT were associated with optimal PFS and OS, respectively. Cam + CT provided the best PFS in male patients with a history of smoking and an ECOG score of 0. In both female and non-smoking patient subgroups, Pem + CT was associated with the best PFS and OS benefits.
Conclusion
For patients with advanced non-selective PD-L1 NSCLC, two effective regimens are Pen + CT and Niv + Bev + CT, which rank first in OS and PFS among all patients. Cam + CT and Tor + CT have advantages for OS in patients with SqNSCLC and Non-sqNSCLC, respectively. Niv + Ipi + CT provided the best OS benefit for patients with an ECOG score of 0, while Pem + CT may be the most effective treatment for patients with an ECOG score of 1. Pem + CT has a better effect on female patients and non-smokers. Sin + CT was found to be the most effective treatment for male patients and the smoking subgroup, while Cam + CT was found to be the most effective for PFS. In addition, Tor + CT was associated with the best PFS for patients with negative PD-L1 expression. Pem + CT was found to significantly improve both PFS and OS compared to CT alone. For patients with positive PD-L1 expression, Sin + CT and Cam + CT were found to be optimal for OS and PFS, respectively. It is important to note that, with the exception of Tor + CT, the toxicity of the other combinations was higher than that of CT alone.






Similar content being viewed by others
References
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41(10):1037–48. https://doi.org/10.1002/cac2.12197.
Kaur J, Elms J, Munn AL, Good D, Wei MQ. Immunotherapy for non-small cell lung cancer (NSCLC), as a stand-alone and in combination therapy. Crit Rev Oncol Hematol. 2021;164:103417. https://doi.org/10.1016/j.critrevonc.2021.103417.
Zhou C, Wang J, Wang B, Cheng Y, Wang Z, Han B, et al. Chinese experts consensus on immune checkpoint inhibitors for non-small cell lung cancer (2020 version)]. Zhongguo Fei Ai Za Zhi. 2021;24(4):217–35. https://doi.org/10.3779/j.issn.1009-3419.2021.101.13.
Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond). 2022;42(10):937–70. https://doi.org/10.1002/cac2.12359.
Judd J, Borghaei H. Combining immunotherapy and chemotherapy for non-small cell lung cancer. Thorac Surg Clin. 2020;30(2):199–206. https://doi.org/10.1016/j.thorsurg.2020.01.006.
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–74. https://doi.org/10.1016/j.csbj.2019.03.006.
Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15(5):1111–22. https://doi.org/10.1080/21645515.2019.1571892.
Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab Plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. 2023;41(11):1999–2006. https://doi.org/10.1200/JCO.22.01990.
Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41(11):1992–8. https://doi.org/10.1200/JCO.22.01989.
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130) : a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–37. https://doi.org/10.1016/S1470-2045(19)30167-6.
Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351–60. https://doi.org/10.1016/j.jtho.2020.03.028.
National Comprehensive Cancer Network. (NCCN) Clinical practice guidelines in oncology. Non small cell lung cancer, version 3. 2023. https://www.nccn.org/profe-ssionals/physician_gls/f_guidelines.asp. Accessed 15 Sep 2023
Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30(6):507–19. https://doi.org/10.1038/s41422-020-0337-2.
Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: a multicenter randomized phase III trial (CHOICE-01). J Clin Oncol. 2023;41(3):651–63. https://doi.org/10.1200/JCO.22.00727.
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (oncology pRogram by InnovENT anti-PD-1–11). J Thorac Oncol. 2020;15(10):1636–46. https://doi.org/10.1016/j.jtho.2020.07.014.
Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen G, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: results from a randomized, double-blind, phase 3 trial (ORIENT-12). J Thorac Oncol. 2021;16(9):1501–11. https://doi.org/10.1016/j.jtho.2021.04.011.
Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed as first-line treatment for advanced nonsquamous NSCLC: extended follow-up of camel phase 3 trial. J Thorac Oncol. 2023;18(5):628–39. https://doi.org/10.1016/j.jtho.2022.12.017.
Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (camel-sq): a phase 3 trial. J Thorac Oncol. 2022;17(4):544–57. https://doi.org/10.1016/j.jtho.2021.11.018.
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512–22. https://doi.org/10.1016/j.jtho.2021.05.005.
Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709–17. https://doi.org/10.1001/jamaoncol.2021.0366.
Han B, Jiao S, Chen J, Wang Z, Zhao Y, Zhang G, et al. 59MO-Final analysis of AK105–302: a randomized, double-blind, placebo- controlled, phase Itrial of penpulimab plus carboplatin andpaclitaxel as first-line treatment for advanced squamous NSCLC. Immuno-Oncology and Technology. 2022;16(1): 100102. https://doi.org/10.1016/j.iotech.2022.100164.
Zhou C, Wang Z, Sun M, Cao L, Ma Z, Wu R, et al. Interim survival analysis of the randomized phase III GEMSTONE-302 trial: sugemalimab or placebo plus chemotherapy as first-line treatment for metastatic NSCLC. Nat Cancer. 2023;4(6):860–71. https://doi.org/10.1038/s43018-023-00578-z.
Awad MM, Gadgeel SM, Borghaei H, Patnaik A, Yang JC, Powell SF, et al. Long-term overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumabas first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol. 2021;16(1):162–8. https://doi.org/10.1016/j.jtho.2020.09.015.
Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351–60. https://doi.org/10.1016/j.jtho.2020.03.028.
Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653–64. https://doi.org/10.1016/j.jtho.2020.11.025.
Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16(11):1909–24. https://doi.org/10.1016/j.jtho.2021.07.009.
Brahmer JR, Lee JS, Ciuleanu TE, Bernabe Caro R, Nishio M, Urban L, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in checkmate 227. J Clin Oncol. 2023;41(6):1200–12. https://doi.org/10.1200/JCO.22.01503.
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. https://doi.org/10.1016/S1470-2045(20)30641-0.
Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2021;32(29):1137–47. https://doi.org/10.1016/j.annonc.2021.06.004.
Zhang L, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Final overall survival data of sintilimab plus pemetrexed and platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC in the Phase 3 ORIENT-11 study. Lung Cancer. 2022;171:56–60. https://doi.org/10.1016/j.lungcan.2022.07.013.
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. https://doi.org/10.1056/NEJMoa1810865.
Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32(7):881–95. https://doi.org/10.1016/j.annonc.2021.04.008.
Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lungcancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23(2):220–33. https://doi.org/10.1016/S1470-2045(21)00650-1.
Kim HR, Sugawara S, Lee JS, Kang JH, Inui N, Hida T, et al. First-line nivolumab, paclitaxel, carboplatin, and bevacizumab for advanced non-squamous non-small cell lung cancer: Updated survival analysis of the ONO-4538–52/TASUKI-52 randomized controlled trial. Cancer Med. 2023;12(16):17061–7. https://doi.org/10.1002/cam4.6348.
Liu L, Bai H, Wang C, Seery S, Wang Z, Duan J, et al. Efficacy and safety of first-line immunotherapy combinations for advanced NSCLC: a systematic review and network meta-analysis. J Thorac Oncol. 2021;16(7):1099–117. https://doi.org/10.1016/j.jtho.2021.03.016.
Wang L, Yang Y, Yu J, Zhang S, Li X, Wu X, et al. Efficacy and safety of anti-PD-1/PD-L1 in combination with chemotherapy or not as first-line treatment for advanced non-small cell lung cancer: a systematic review and network meta-analysis. Thorac Cancer. 2022;13(3):322–37. https://doi.org/10.1111/1759-7714.14244.
Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–91. https://doi.org/10.1172/JCI80011.
Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res. 2017;23(12):3158–67. https://doi.org/10.1158/1078-0432.
Zhao M, Shao T, Ren Y, Zhou C, Tang W. Identifying optimal PD-1/PD-L1 inhibitors in first-line treatment of patients with advanced squamous non-small cell lung cancer in China: updated systematic review and network meta-analysis. Front Pharmacol. 2022;13:910656. https://doi.org/10.3389/fphar.2022.910656.
Shao J, Wang C, Ren P, Jiang Y, Tian P, Li W. Treatment- and immune-related adverse events of immune checkpoint inhibitors in advanced lung cancer. Biosci Rep. 2020;40(5):20192347. https://doi.org/10.1042/BSR20192347.
Mohammed N, Xiao EH, Mohsen S, Xiong Z, Zhou R. PD-1/PD-L1 inhibitor treatment and its impact on clinical imaging in non-small cell lung cancer: a systematic review and meta-analysis of immune-related adverse events. Front Oncol. 2023;13:1191681. https://doi.org/10.3389/fonc.2023.1191681.
Wang Z, Zhao J, Ma Z, Cui J, Shu Y, Liu Z, et al. A phase 2 study of tislelizumab in combination with platinum-based chemotherapy as first-line treatment for advanced lung cancer in chinese patients. Lung Cancer. 2020;147:259–68. https://doi.org/10.1016/j.lungcan.2020.06.007.
Yang JJ, Huang C, Fan Y, Pan H, Feng J, Jiang L, et al. Camrelizumab in different PD-L1 expression cohorts of pre-treated advanced or metastatic non-small cell lung cancer: a phase II study. Cancer Immunol Immunother. 2022;71(6):1393–402. https://doi.org/10.1007/s00262-021-03091-3.
Pérez-Ruiz E, Melero I, Kopecka J, Sarmento-Ribeiro AB, García-Aranda M, De Las RJ. Cancer immunotherapy resistance based on immune checkpoints inhibitors: Targets, biomarkers, and remedies. Drug Resist Updat. 2020;53:100718. https://doi.org/10.1016/j.drup.2020.100718.
Lu Y, Zhang X, Ning J, Zhang M. Immune checkpoint inhibitors as first-line therapy for non-small cell lung cancer: a systematic evaluation and meta-analysis. Hum Vaccin Immunother. 2023;19(1):2169531. https://doi.org/10.1080/21645515.2023.2169531.
Siciliano MA, Caridà G, Ciliberto D, d’Apolito M, Pelaia C, Caracciolo D, et al. Efficacy and safety of first-line checkpoint inhibitors-based treatments for non-oncogene-addicted non-small-cell lung cancer: a systematic review and meta-analysis. ESMO Open. 2022;7(3):100465. https://doi.org/10.1016/j.esmoop.2022.100465.
Mo DC, Huang JF, Luo PH, Huang SX, Wang HL. The efficacy and safety of combination therapy with immune checkpoint inhibitors in non-small cell lung cancer: A meta-analysis. Int Immunopharmacol. 2021;96:107594. https://doi.org/10.1016/j.intimp.2021.107594.
Peng TR, Lin HH, Tsai FP, Wu TW. Immune checkpoint inhibitors for first-line treatment of advanced non-small-cell lung cancer: a systematic review and network meta-analysis. Thorac Cancer. 2021;12(21):2873–85. https://doi.org/10.1111/1759-7714.14148.
Liu T, Wu S, Fang W, Li H, Su L, Qi G, et al. Identifying optimal first-line immune checkpoint inhibitors based regiments for advanced non-small cell lung cancer without oncogenic driver mutations: a systematic review and network meta-analysis. PLoS ONE. 2023;18(4):e0283719. https://doi.org/10.1371/journal.pone.0283719.
Huang Z, Pang X, Zhong T, Qu T, Chen N, Ma S, et al. Penpulimab, an fc-engineered igG1 anti-PD-1 antibody, with improved efficacy and low incidence of immune-related adverse events. Front Immunol. 2022;13:924542. https://doi.org/10.3389/fimmu.2022.924542.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflict interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Chen, W., Dong, L. et al. Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03442-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03442-3