Abstract
Background
Mesenchymal epithelial transition factor (MET) is a rare oncologic driver gene, and information on immunotherapy for non-small cell lung cancer (NSCLC) patients with this driver gene is limited. Here we evaluate the efficacy and safety of immune checkpoint inhibitors (ICI) under different therapeutic regimen for NSCLC patients with MET alterations.
Methods
From June 2019 to December 2023, we assessed the efficacy and toxicity of ICIs in 42 NSCLC patients with MET alterations. Survival curves were plotted using the Kaplan–Meier method and the Cox proportional hazards model applied for univariate and multivariate analyses. We assessed the size of target lesion according to RECIST v1.1, and objective response rate (ORR) was defined as the sum of complete response (CR) and partial response (PR), disease control rate (DCR) as the sum of CR, PR, and disease stable.
Results
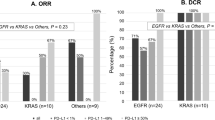
A total of 42 NSCLC patients with MET alterations were included in this retrospective study, 10 was MET 14 skipping mutation and 32 was MET amplification. The ORR for ICI treatment was 30.95% and the DCR was 71.43%. Median progression-free survival (mPFS) and median overall survival (OS) were 4.40 and 13.97 months, respectively. There exists statistical differences between the mPFS of ICI monotherapy and combine ICI therapy (2.8 vs 7.8 months, p = 0.022). The incidence of drug-related adverse reactions was 47.62%, mainly bone marrow suppression (14.28%), immune-related pneumonia (7.14%), and liver function impairment (7.14%), and six patients (14.28%) experiencing grade 3 or above adverse events.
Conclusion
NSCLC patients with MET alterations can benefit from immunotherapy, especially the patients treated by combined ICI therapy. However, special attention should be paid to the occurrence of grade 3/4 adverse reactions while using the combined ICI therapy.



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135(5):584–90. https://doi.org/10.1097/CM9.0000000000002108.
Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. 2018;6(5):e555–67. https://doi.org/10.1016/S2214-109X(18)30127-X.
Sholl LM, Aisner DL, Varella-Garcia M, Berry LD, Dias-Santagata D, Wistuba II, et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol. 2015;10(5):768–77. https://doi.org/10.1097/JTO.0000000000000516.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. https://doi.org/10.3322/caac.21565.
Wen S, Dai L, Wang L, Wang W, Wu D, Wang K, et al. Genomic signature of driver genes identified by target next-generation sequencing in Chinese non-small cell lung cancer. Oncologist. 2019;24(11):e1070–81. https://doi.org/10.1634/theoncologist.2018-0572.
Favoni RE, Alama A. Preclinical strategies targeted at non-small-cell lung cancer signalling pathways with striking translational fallout. Drug Discov Today. 2013;18(1–2):11–24.
Engelman J. C-MET, a marker of resistance to EGFR inhibitors in NSCLC. Ann Oncol. 2008;19:22. https://www.researchgate.net/publication/295962404_C-Met_a_marker_of_resistance_to_EGFR_inhibitors_in_NSCLC.
Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019;16:341–55. https://doi.org/10.1038/s41571-019-0173-9.
Dantoing E, Piton N, Salaün M, Thiberville L, Guisier F. Anti-PD1/PD-L1 immunotherapy for non-small cell lung cancer with actionable oncogenic driver mutations. Int J Mol Sci. 2021;22(12):6288. https://doi.org/10.3390/ijms22126288.
Vuong HG, Ho ATN, Altibi AMA, Nakazawa T, Katoh R, Kondo T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer—a systematic review and meta-analysis. Lung Cancer. 2018;123:76–82. https://doi.org/10.1016/j.lungcan.2018.07.006.
Schrock AB, Frampton GM, Suh J, Chalmers ZR, Rosenzweig M, Erlich RL, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493–502. https://doi.org/10.1016/j.jtho.2016.06.004.
Robinson KW, Sandler AB. The role of MET receptor tyrosine kinase in non-small cell lung cancer and clinical development of targeted anti-MET agents. Oncologist. 2013;18:115–22.
Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–57. https://doi.org/10.1056/NEJMoa2002787.
Yang JJ, Fang J, Shu YQ, Chang JH, Chen GY, He JX, et al. A phase Ib study of the highly selective MET-TKI savolitinib plus gefitinib in patients with EGFR-mutated, MET-amplified advanced non-small-cell lung cancer. Invest New Drugs. 2021;39(2):477–87. https://doi.org/10.1007/s10637-020-01010-4.
Camidge DR, Otterson GA, Clark JW, Ignatius Ou SH, Weiss J, Ades S, et al. Crizotinib in patients with MET-amplified NSCLC. J Thorac Oncol. 2021;16(6):1017–29. https://doi.org/10.1016/j.jtho.2021.02.010.
Seto T, Ohashi K, Sugawara S, Nishio M, Takeda M, Aoe K, et al. Capmatinib in Japanese patients with MET exon 14 skipping-mutated or MET-amplified advanced NSCLC: GEOMETRY mono-1 study. Cancer Sci. 2021;112(4):1556–66. https://doi.org/10.1111/cas.14826.
Dagogo-Jack I, Moonsamy P, Gainor JF, Lennerz JK, Piotrowska Z, Lin JJ, et al. A phase 2 study of capmatinib in patients with MET-altered lung cancer previously treated with a MET inhibitor. J Thorac Oncol. 2021;16(5):850–9. https://doi.org/10.1016/j.jtho.2021.01.1605.
Li J, Wang Y, Zhang B, Xu J, Cao S, Zhong H. Characteristics and response to crizotinib in lung cancer patients with MET amplification detected by next-generation sequencing. Lung Cancer. 2020;149:17–22. https://doi.org/10.1016/j.lungcan.2020.08.021.
Yu Y, Zhou J, Li X, Goto K, Min X, Nishino K, et al. Gumarontinib in patients with non-small-cell lung cancer harbouring MET exon 14 skipping mutations: a multicentre, single-arm, open-label, phase 1b/2 trial. EClinicalMedicine. 2023;6(59): 101952. https://doi.org/10.1016/j.eclinm.2023.101952.
Drilon A, Clark JW, Weiss J, Ou SI, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26(1):47–51. https://doi.org/10.1038/s41591-019-0716-8.
Landi L, Chiari R, Tiseo M, D’Incà F, Dazzi C, Chella A, et al. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non-small cell lung cancer (METROS): a phase II, prospective, multicenter, two-arms trial. Clin Cancer Res. 2019;25(24):7312–9. https://doi.org/10.1158/1078-0432.CCR-19-0994.
Le X, Sakai H, Felip E, Veillon R, Garassino MC, Raskin J, et al. Tepotinib efficacy and safety in patients with MET exon 14 skipping NSCLC: outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clin Cancer Res. 2022;28(6):1117–26. https://doi.org/10.1158/1078-0432.CCR-21-2733.
Schuler M, Berardi R, Lim WT, de Jonge M, Bauer TM, Azaro A, et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: clinical and biomarker results from a phase I trial. Ann Oncol. 2020;31(6):789–97. https://doi.org/10.1016/j.annonc.2020.03.293.
Lu S, Fang J, Li X, Cao L, Zhou J, Guo Q, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: a multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med. 2021;9(10):1154–64. https://doi.org/10.1016/S2213-2600(21)00084-9.
Guisier F, Dubos-Arvis C, Viñas F. Efficacy and safety of anti-PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol. 2020;15:628–36. https://doi.org/10.1016/j.jtho.2019.12.129.
Sabari JK, Leonardi GC, Shu CA, Umeton R, Montecalvo J, Ni A, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29(10):2085–91. https://doi.org/10.1093/annonc/mdy334.
Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–8.
Zhang Y, Yang Q, Zeng X, Wang M, Dong S, Yang B, et al. MET amplification attenuates lung tumor response to immunotherapy by inhibiting STING. Cancer Discov. 2021;11(11):2726–37. https://doi.org/10.1158/2159-8290.CD-20-1500.
Chen B, Wang J, Pu X, Li J, Wang Q, Liu L, et al. The efficacy and safety of immune checkpoint inhibitors combined with chemotherapy or anti-angiogenic therapy as a second-line or later treatment option for advanced non-small cell lung cancer: a retrospective comparative cohort study. Transl Lung Cancer Res. 2022;11(10):2111–24. https://doi.org/10.21037/tlcr-22-697.
Schoenfeld AJ, Rizvi H, Bandlamudi C, Sauter JL, Travis WD, Rekhtman N, et al. Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Ann Oncol. 2020;31:599–608.
Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:4226. https://doi.org/10.1136/bmj.k4226.
Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol. 2020;10:91. https://doi.org/10.3389/fonc.2020.00091.
Acknowledgements
The authors would like to appreciate all patients and their families for their cooperation and participation. Additionally, we are thankful to all research staff and co-investigators involved in this research.
Funding
The study was supported by the Medical Scientific Research Foundation of Zhejiang Province (No. 2022KY653), and sponsored by Zhejiang provincial program for the Cultivation of High-Level Innovative Health Talents (to Zhengbo Song).
Author information
Authors and Affiliations
Contributions
ZS and YH designed and supervised the research. YW, JW, MX, JX and KS conducted the follow-up, data collection, and correlative analysis. YW, MX and YH provided support in data analysis and use of software. YW, JW, MX and KS provided data analysis. YW, MX and YH were involved in manuscript preparation and all the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethics approval and consent to participate
Approval of the study protocol was obtained from Zhejiang Cancer Hospital Institutional Review Board Committee (approval number: IRB-2022-396). Individual consent for this retrospective analysis was waived.
Consent for publication
Not applicable.
Consent to participate and publish
Individual consent for analysis was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
See Fig.
4.
Appendix 2
See Fig.
Kaplan–Meier estimates of progression-free survival (PFS) and overall survival (OS). a PFS of three type patients with MET alterations (MET exon 14 skipping vs primary amplification vs secondary amplification: 9.2 vs 7.8 vs 3.4 months, p = 0.645); b OS of three type patients with MET alterations (MET exon 14 skipping vs primary amplification vs secondary amplification: 16.7 vs 15.1 vs 10.8 months, p = 0.493)
5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Wei, J., Xu, M. et al. Efficacy and safety analysis of immunotherapy in non-small cell lung cancer patients with MET alterations. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03455-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03455-y