Abstract
Traditional corrosion inhibitors have been frequently used for metal protection against strong acids. However, most of them are hazardous compounds with high costs. Therefore, due to the strict environmentally friendly regulations, green and low-cost compounds from renewable sources have gained high consideration in recent applications. In the present study, the Arachis hypogaea shell extract, commonly called groundnut, a cost-effective was selected to inhibit API X 65 pipeline steel corrosion in 1 mol·L−1 H2SO4 solution. The Arachis hypogaea shell composition was examined by FTIR analysis. The Arachis hypogaea shell on pipeline steel in H2SO4 solutions was studied via gravimetric, potentiodynamic polarization, and surface analysis (SEM and EDX). Electron donor atoms present in the Arachis hypogaea shell extract molecules is responsible for its adsorption on the surface of the metal. Experimental study shows that with increasing Arachis hypogaea shell concentrations, the inhibition tendency increased and reached 98.84% at 4 g L−1 after 24 h. Arachis hypogaea shell extract behaved as mixed-type inhibitor, and the surface coverage fits the Langmuir isotherm, signifying that the steel surface was covered by a monolayer of inhibitor molecules without intermolecular interactions. SEM analysis shows that Arachis hypogaea molecule adsorption on the metal surface reduced considerably its dissolution rate resulting in a smooth and clean surface with few damaged areas. The use of water for extraction of Arachis hypogaea shell exhibiting inhibition efficiency, non-toxic, and cost-effective than other green inhibitors, proposes the Arachis hypogaea shell as an excellent green inhibitor for API X 65 steel corrosion in 1 mol·L−1 H2SO4 solution.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
API X 65 pipeline steel is characterized by its excellent weldability, toughness, and tensile strength. It is used in the oil and gas sector as one of the primary steels used in pipes [1–3]. The API X 65 pipeline steel is now highly considered in industrial development due to its strength and low cost [4–7]. Acid cleaning, a typical pickling and de-scaling process in industrial application, via strong acid, that is, H2SO4, HCl, H3PO4, and HNO3 causes metallic structures corrosion [8–11]. Material loss due to corrosion leads to undesirable defects and failures. Corrosion is a spontaneous and irreversible deterioration of alloys or metals via electrochemical or chemical reactions with the environments, leading to material wastes and enormous economic. Corrosion can be delayed or controlled in different ways, for instance, corrosion inhibitors, cathodic protection, and surface coatings. Corrosion inhibitors, classified as inorganic and organic group, is one of the most cost-effective techniques of controlling corrosion [12, 13]. In acidic solutions, the adsorption of the organic compounds on the metal surface protects it from corroding.
Traditional inhibitor such as azoles is usually utilized for this purpose, not only because of negative environmental impacts, but also due to its high cost and toxicity. Their application in most fields, including engineering, science, and technology has been restricted. Recently, research interests have been directed toward replacing expensive synthetic organic inhibitors and toxic inhibitors with non-toxic inhibitors like oil [14], plant extracts [15, 16], drugs [17, 18], ionic liquids [19, 20], and biopolymers [21, 22]. In selecting green inhibitor, the most critical parameter that should be considered are environmental issue, inhibition capacity, availability, cost, and extraction process. In general, sustainable and green inhibitors based on ionic liquids, drugs, and biopolymers are expensive compounds to be utilized in acid solutions for controlling metal against the corrosion. Though, various plants parts are renewable source of inexpensive green inhibitor. There are numerous environmentally friendly and cost-effective means for extracting various plants species. The plant extract is rich in different efficient organic compound, including, oxygen and nitrogen, aromatic rings, heterocyclic rings, and aliphatic chains. In view of this, plants extracts can adsorb effectively on the surface of the metal and protects the metal from corroding with insignificant negative impacts on the environments [23]. There are several reports on the utilization of plant extract in mitigating metal in aggressive solutions using green inhibitors from plant sources. Nevertheless, attaining high inhibition efficiency is the main target in most of these reports, and ignoring the economic aspect. Several reports used organic solvent such as methanol and ethanol in extracting these inhibitors. Utilizing this solvent makes the process not eco-friendly and expensive.
Popoola et al investigated the influence of avocado seed extracts on the corrosion of API X 65 pipeline steel in 1 M H2SO4 solutions and found 88% efficiency with 5 g L−1 inhibitor [24]. Amal A Altalhi showed that diazene-based Schiff base derivatives showed 90.4 and 92.6% efficiency on API stainless steel at 100 ppm concentration [25]. In another effort, Alao et al used 5 g Persea Americana seed extract in 1 M HCl media and achieved 95.65% efficiency [26]. Abeng et al demonstrated that gentamicin and sulfamethoxazole served as a suitable inhibitor for API 5 L X-52 pipeline steel in hydrochloric acid solutions with 90% and 80% efficiency, respectively, at 500 ppm [27]. In another study, Espinoza-Vázquez et al observed that using Trasar Trac102, 95.4% inhibition efficiency was achieved for API 5 L X65 steel under static conditions at 20 ppm in NaCl 3% wt. [28]. It was observed that most of the selected inhibitors for mitigating pipeline steel in the corrosive solutions gives inhibition efficiency between 85 and 95%. In addition, the extraction solvents commonly used are methanol and ethanol, which are not environmentally safe and the green extracts utilized are expensive. Therefore, effective inhibition of API X 65 pipeline steel in corrosive solutions with readily available substances in high quantity from low-cost sources are crucial factor to be considered when selecting green inhibitors. Therefore, Arachis hypogaea green source and cost-effective corrosion inhibitor was introduced in this study.
Groundnut is one of the main seed and economic crop of the world. It is used as fodder for livestock, green manure, and for human consumption as protein and vegetable oil. With about 48% oil, 3% fiber, and 26% protein, with high contents of niacin, calcium, and thiamine, it can be utilized as an economical food supplement to fight malnutrition. There are eight fatty acids in groundnut, viz. lignoseric (24:0), behenic (22:0), arachidic (20:0), palmitic (16:0), stearic (18:0), linoleic (18:2), eicosenoic (20:1), and oleic (18:1) [29]. The price of 1 kg Arachis hypogaea shell is R0.00 in South Africa, the shell is regarded as a waste. Meaning it is cheap, and extracting the inhibitor molecule from this shell would have many economic and environmental benefits. Arachis hypogaea shell extracts was applied in this study, as cost-effective and environmentally friendly inhibitor for pipeline steel in H2SO4 solutions. The component present in the Arachis hypogaea shell extract (AHS) was examined by FTIR analysis. The AHS corrosion inhibition efficiency and corrosion inhibition mechanisms was studied on API X 65 pipeline steel in 1 mol L−1 H2SO4 solutions by gravimetric, and potentiodynamic polarization test. Surface analysis was carried out by SEM/EDX analysis.
2. Experimental
2.1. Material and sample preparation
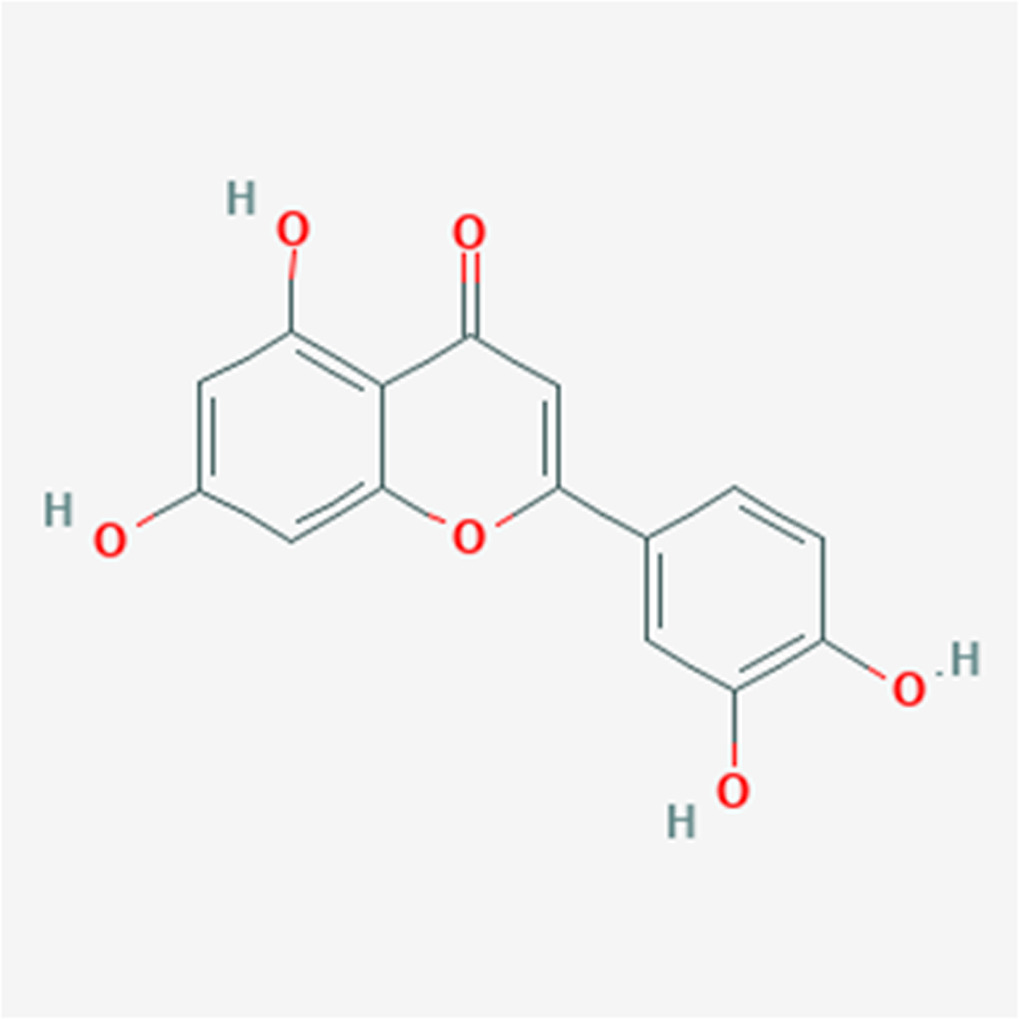
For corrosion studies, the steel specimen utilized was API X 65 pipeline steel with the following compositions (%): Si 0.186, C 0.09, Ni 0.016, Nb 0.023, Cu 0.01, Al 0.016, Mn 0.867, P 0.006, S 0.003 and Fe Balance. A 1 mol L−1 H2SO4 solutions was prepared by diluting analytical grade H2SO4 and used as the electrolytic solution. With different grades of emery papers, the scale and oxide was cautiously rubbed from the steel surface, dipped in acetone to remove the organic contamination, and dried, then stored in moisture-free desiccators. The Arachis hypogaea shell was collected from Gauteng, South Africa. The extract was dried and weighed, and the shell was powdered for the extraction process. Afterward, 1 L distilled water was refluxed to 40 g of Arachis hypogaea shell dry powder, the resulting solution was continuously stirred at 70 ◦C for 3 h. The solution was then passed from the filter to separate the undesirable parts. The clear solution was centrifuged at 4500 rpm power for 20 min. Then, the solution was heated at 70 ◦C for 24 h, and the un-dissolved specie was separated by a filter. Numerous solvents can be used in extracting green inhibitors. Nevertheless, water solvent, which is eco-friendly and cheaper than other solvents ( i.e., cyclohexane, methanol, and ethanol), was used in this study. Also, only the component totally soluble in 1 mol L−1 H2SO4 solution is essential. A specific quantity of Arachis hypogaea shell extracts was dissolved in test solutions for gravimetric, morphological, and electrochemical studies. The chemical structures of Arachis hypogaea is displayed in figure 1.
Figure 1. Chemical structure of Arachis hypogaea.
Download figure:
Standard image High-resolution image2.2. Methods
2.2.1. Potentiodynamic polarization measurements
The potentiodynamic polarization test was carried out in a three-electrode cell, where the Ag/AgCl. Sat, platinum rod, and API X 65 pipeline steel were used as reference, counter, and working electrodes. The surface area of the working electrode exposed to the 1 mol L−1 H2SO4 solutions was 1 cm 2. Before the experiments, the working electrode was abraded with 220, 600, 800, and 1200-grit SiC papers, ultrasonically cleaned with deionized water, degreased with acetone, and dried. The experiment was conducted in triplicates with new samples. The measurements were carried out in the 1 mol L−1 H2SO4 solutions without and with 0, 1, 2, 3, 4, and 5 g/l AHS. In the polarization tests, the potential was scanned at ±250 mV at a scan rate of 1 mV s−1. The experiment was performed at 25 °C. Each experiment was conducted three times, to ensure suitable reproducibility.
2.2.2. Gravimetric measurements
Weight loss was measured for 456 h exposure time in 1 mol L−1 H2SO4 solutions protected by AHS (0, 1, 2, 3, 4, and 5 g L−1). At 24 h intervals, the specimen was removed, washed with distilled water to remove corrosion product, dried, and weighed carefully. Equation (1) was used to compute the average weight loss of the un-inhibited and inhibited samples [30]:
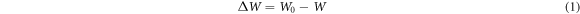
Where W0 and W are the steel weight before and after immersion
The corrosion rate (CR) was estimated using equation (2):
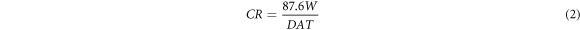
Also, the inhibition efficiency was estimated using equation (3):
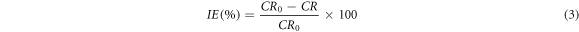
Where, CR0 and CR is the corrosion rate of uninhibited and inhibited sample.
2.2.3. Surface characterizations
FTIR analysis was carried out by the Perkin Elmer instrument to confirm the functional groups present in the AHS molecule. This was carried out on the pellet of KBr mixed powder with AHS extracts in 400–4000 cm−1 wave number. The SEM images were taken to understand the surface morphology of the steel surface exposed to H2SO4 solutions with 4 g L−1 of AHS.
3. Result and discussion
3.1. Potentiodynamic polarization measurements
The effects of AHS on the cathodic and anodic reactions was established by potentiodynamic polarization study in 1 mol L−1 H2SO4 solution and the obtained results are shown in figure 2. By extrapolating the cathodic and anodic part of polarization curve, the corrosion current densities, icorr, anodic Tafel slopes, βa, corrosion potentiasl, Ecorr, and cathodic Tafel slopes, βc, were achieved and listed in table 1. It is evident from the data given in figure 2 that by using AHS, the corrosion current density and the corrosion rate were reduced significantly compared to the uninhibited sample. From the polarization curves, the major shift of cathodic and anodic branches to lower current densities values is obvious signifying that the AHS reduced the cathodic and anodic corrosion reactions via mixed inhibition mechanisms. The displacement in the Ecorr towards more negative value is lower than ±85 mV as shown in table 1, signifying that the AHS behaves as mixed inhibitor [31]. The cathodic branch of the inhibited and uninhibited sample is approximately parallel, denoting the adsorption of the inhibitor on the surface of the metal on the hydrogen evolution reactions on cathodic site [32]. Conversely, the iron dissolution mechanisms was influenced with the inhibitor via observation of noticeable change in anodic branch slope and shape [33]. The impacts of AHS on the corrosion of X 65 steel can be observed from the obvious decline in icorr with 4 g/l AHS. The polarization plots results show the AHS's high efficiency block the X 65 steel reaction site. As shown in table 1, corrosion rate and icorr declines denote the effect of the AHS layer against steel corrosion with increasing AHS concentrations [34]. Conversely, the change in βa value is principally because of the SO4 2—ions adsorbed from corrosive solution to the metal substrate.
Figure 2. Potentiodynamic polarization curves for API X 65 steel after 5 h in H2SO4 solutions with various concentration of AHS.
Download figure:
Standard image High-resolution imageTable 1. Potentiodynamic polarization data's after 5 h for API X 65 steel in H2SO4 with various concentration of AHS.
| Inhibitor concentration (g/L) | βa (V/dec) | βc (V/dec) | Ecorr (V) | Icorr (A/cm2) | Polarization resistance (Ω) | Corrosion rate (mm/year) |
|---|---|---|---|---|---|---|
| Blank | 0.0424 | 0.1612 | −0.3654 | 0.0007 | 22.58 | 7.5068 |
| 1 | −0.5206 | 0.2136 | −0.4461 | 0.0005 | 339.26 | 5.3860 |
| 2 | 0.4411 | −0.6551 | −0.3626 | 2.34E-05 | 2505.40 | 0.2720 |
| 3 | 0.1008 | 0.1439 | −0.1288 | 2.2E-06 | 11704.00 | 0.0256 |
| 4 | 0.2940 | 0.3302 | −0.1933 | 1.49E-06 | 45341.00 | 0.0173 |
| 5 | 0.6887 | 0.6072 | −0.3833 | 8.54E-05 | 1640.20 | 0.9928 |
3.2. Gravimetric measurements
The plot of the weight loss, corrosion rate, degree of surface coverage and inhibition efficiency were summarized in figure 3. Evidence from figure 3 proved that after adding the AHS to the aggressive solutions, the steel corrosion significantly reduced. By increasing the AHS concentrations, the corrosion rate was suppressed, and the inhibition performance was enhanced. This increase could be ascribed to the AHS molecules adsorbed on the surface active site [35]. The corrosion rates of the uninhibited samples are much higher than the inhibited sample, as shown in figure 3.
Figure 3. Plots of calculated values of (a) weight loss, (b) corrosion rate, (c) degree of surface coverage and (d) inhibition efficiency for API X 65 steel in 1 mol L−1 H2SO4 solutions with different concentration of AHS.
Download figure:
Standard image High-resolution imageAlso, by increasing the exposure time, the value increased remarkably. These increases ensure that the uninhibited samples were corroded severely. Though, the corrosion rate diminished remarkably by increasing the AHS concentration in the aggressive solution. This reduction is directly ascribed to the AHS molecule's adsorbed on the steel active site. Also, increasing the AHS concentrations can enhance the surface coverage and improve the performance of higher inhibition [36]. According to figure 3, after adding 4 g/L AHS in the acidic solutions, the rate of corrosion value reduced from 69.88 to 7.59 mm/year. The inhibition performance increased to 98.42% for the specimen in H2SO4 solutions with 4 g/L AHS.
The corrosion rate value was also determined at various AHS concentrations (1, 2, 3, 4, and 5 g L−1). Figure 3 reflects that the corrosion rate was remarkably enhanced by increasing the AHS concentrations. The computed corrosion rate value for the uninhibited sample is higher than the inhibited samples (particularly at 4 g L−1). As discussed previously, the uninhibited sample surface was covered by corrosion product (iron oxides), which can rarely protect the steel from corroding. Nevertheless, with immersion time, iron oxides can easily detach from metal substances.
Therefore, the steel substrates can be highly corroded by aggressive ion attack, resulting in notably increase in the corrosion rate value. Conversely, the corrosion rate did not change considerably at a concentration above 4 g L−1. This observation denotes that the AHS molecule can protects the steel surface from corrosion even at low concentrations [37]. This finding supports that the AHS act via geometric blockage of the metal surface through adsorption mechanisms.
3.3. Arachis hypogaea shell extracts adsorption mechanism
Inhibitor molecule adsorption can be influenced by the inhibitor molecule size, active site number, charge densities, metal surface interaction, and the film stability formed on the metal surface. The molecule adsorbed can bond with the surface of the metal via different mechanism. Accordingly, the organic molecules of Arachis hypogaea can contribute to interaction with metal surfaces via the electron sharing between the electron pair heteroatoms, the vacant iron atoms orbital, and π-electrons. Additionally, inhibitors molecule can interacts with the surface of the metal through van der Waals force and hydrogen bonding mechanism. The isotherms in the Arachis hypogaea shell extracts adsorption on the steel surface was also investigated. Various models, including Frumkin Langmuir, Temkin, and Freundlich was utilized in describing the interactions between AHS molecule and steel. The equation related to this isotherm is as follows:
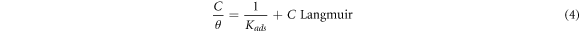
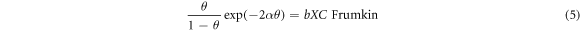
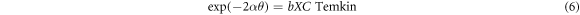
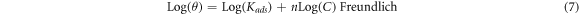
In the presented isotherms, θ, C, and Kads denotes the surface coverage, the Arachis hypogaea shell extracts concentrations in H2SO4 media, and kads is the adsorption equilibrium constant, respectively. Figure 4 illustrates that the best-fitted isotherm is a linear connection between C/θ versus C (g/L) with a 0.99996 slope value. Hence, the AHS adsorption on the steel surface fits the Langmuir isotherms, and a protected monolayer was generated on the steel surface, leading to a wholly smooth surface [38]. The increased Kads with inhibitor concentrations suggest the presence of inhibitor adsorption on the steel surface [39].
Figure 4. (a) Langmuir, (b) Frumkin, (c) Temkin and (d) Freundlich adsorption isotherm for API X 65 steel in H2SO4 solutions with various concentration of AHS.
Download figure:
Standard image High-resolution image3.4. Surface studies
Figure 5 shows the SEM and EDX result for the API X 65 steel surface after 456 h exposure time in 1 mol L−1 H2SO4 solutions inhibited by AHS with 4 g L−1 concentration. The SEM image shown in figure 5 reflects that the AHS makes the steel surface smooth with few corroded areas and damages. Observation from figure 5 shows that without AHS, severe damage, with pit and cavity, showed on the steel surface. This illustration discloses that the passive films covered the steel surface effectively and blocked the active site [40]. It is obvious from this result that the AHS molecules adsorbed on the steel substrates results to a reduced roughness on the metal surface [41, 42]. The reduced surface roughness is obvious from the SEM image in figure 5 with inhibitor. The lower surface roughness indicates the AHS molecules' efficiency in reducing iron dissolution rates. This observation is in close accordance with the gravimetric and electrochemical test result. This result proves the role of Arachis hypogaea shell molecule adsorption on the API X 65 steel surface and its efficiency in iron dissolution rate reduction.
Figure 5. SEM/EDX micrographs for API X 65 steel after 456 h in H2SO4 solutions (a) as-received sample (b) without Arachis hypogaea shell extract, and (c) with 4 g L−1 of Arachis hypogaea shell extract.
Download figure:
Standard image High-resolution image3.5. FTIR analyses of Arachis hypogaea shell extract
As mentioned in the literature calcium, thiamine, and niacin are three significant components present in the Arachis hypogaea. The main absorption band in this compound is −OH, C=O, and −CH3. The organic molecule adsorption in the Arachis hypogaea extract on the API X 65 steel surface depends on the inhibiter molecules chemical structure. To figure out the presence of this band in the Arachis hypogaea shell extracts, the FTIR analysis was performed. From figure 6, the absorption band located at 2355 cm−1 is linked to the –OH stretching vibration. The C=C stretching and –CH3 bending vibration is observed at wavenumber 1606 cm−1 and 1012 cm−1, respectively. The absorption peak at 3390 cm−1 is associated with alkane stretching, respectively. This finding reflects that all significant bonds and functional groups in Arachis hypogaea shell extract are observable from the FTIR spectrum.
Figure 6. FTIR spectrum for Arachis hypogaea shell extract for 400–4000 cm−1 wavenumber.
Download figure:
Standard image High-resolution image3.6. Comparative efficiency and economic studies
Recently, corrosion inhibitors have become extremely common in industrial application. On the other hand, in some cases, this inhibitor can negatively impact the environment and human health. Also, conventional and synthetic inhibitors are generally not cost-effective and eco-friendly. Hence, scientists now focus on green corrosion inhibitors. Owing to its adverse impacts and utterly toxic nature on human health and the environment, the application of inhibitor such as chromate has been limited, and environmentally friendly inhibitor such as seeds of fruits, roots, leaves of plants and shells has received much attention as possible replacements. Organic compound with active functional groups and heteroatoms provide high inhibitive effectiveness for green inhibitors. In this study, from the gravimetric test with 4 g/L Arachis hypogaea shell extract, 98.42% efficiency was derived. This efficiency is greater than most of the reported green inhibitors [24–27]. The process of extracting most inhibitors with efficiency lower than Arachis hypogaea shell extracts are expensive. 1 kg of Arachis hypogaea shell extracts needs only R0.00, while most green inhibitors' extraction process is higher than $50 per kg.
Conversely, high-performance inhibitor such as Ceratonia siliqua L seed oil [43–45], and Polysaccharide [46–48] are costly and, hence, are not recommended for large scale because of high preparation prices. It is worth noticing that most inhibitors reached highest performance at high concentration, increasing the related cost. Also, extraction of Arachis hypogaea shell is green extraction, non-aqueous solvent (for instance, methanol cyclohexane, and ethanol) are essential to extract most green inhibitors, which leaves negative impacts on the environmental and increase the cost also. Most of the reported environmentally friendly inhibitors are scarce, which restricts the extensive utilization of the inhibitors. Hence, the Arachis hypogaea shell extracts can be used for large-scale application because of its high availability, low cost, high performance, and eco-friendly nature.
4. Conclusion
The effect of Arachis hypogaea shell extract on the API X 65 steel corrosion in H2SO4 solutions was studied via gravimetric, surface characteristic and electrochemical technique. FTIR analysis demonstrated the presence of active components in the Arachis hypogaea shell extracts. The presence of functional groups that is, −OH, C=O, –CH3, in the Arachis hypogaea shell was shown by the FTIR test. The SEM and EDX morphological observations showed that the steel surface became smoother with inhibitor. The surface damage due to the iron severe dissolution in the H2SO4 solution was decreased remarkably by adding 4 g/l Arachis hypogaea shell extract. Based on gravimetric test results, the result clarified that with increase in the AHS concentration, an optimum efficiency of 98.42% was achieved with 4 g/l Arachis hypogaea shell extract after 24 h. One novel achievement from this study compared to the previous ones is that most green and synthetic corrosion inhibitors used for pipeline steel protection in H2SO4 solutions did not provide high efficiency at long exposure time. The green compound of Arachis hypogaea shell extracts provided high inhibition efficiency even at a long exposure time (65.60 after 432 h). Potentiodynamic measurement proved that the Arachis hypogaea shell extracts act as mixed-type. The adsorption of Arachis hypogaea shell extract on the metal surface, obeys the Langmuir isotherms. Comparing conventional synthetic corrosion inhibitors with the Arachis hypogaea shell extract, Arachis hypogaea shell extract is non-toxic compound with similar efficiency and low cost. We could recommend using this Arachis hypogaea shell extracts in H2SO4 solutions for acid-pickling process used in oil and gas pipeline surface preparation based on the derived result.
Acknowledgments
The authors appreciate the Surface Engineering Research Laboratory (SERL) at Tshwane University of Technology, Pretoria, for their Laboratory apparatus and official assistance.
Data availability statement
The data cannot be made publicly available upon publication because the cost of preparing, depositing and hosting the data would be prohibitive within the terms of this research project. The data that support the findings of this study are available upon reasonable request from the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.