Abstract
We present the structural, elastic, electronic, magnetic, and phonon properties of D0c X3Ru (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn) alloys in their respective ground-states at zero pressure using first-principles density functional theory (DFT). The calculated heat of formation for Sc3Ru, Ti3Ru, V3Ru, Mn3Ru, and Zn3Ru are negative, signifying their thermodynamic stability. Meanwhile, we find that Sc3Ru, V3Ru, Mn3Ru, Co3Ru, Ni3Ru, Cu3Ru and Zn3Ru alloys are mechanically stable. The electronic properties indicate a metallic nature in all the X3Ru alloys due to valence-conduction band overlap at the Fermi energy. Additionally, the phonon dispersion curves suggest that Cr3Ru, Fe3Ru, Ni3Ru, Cu3Ru, and Zn3Ru are dynamically stable. These results provide a comprehensive overview of the stability, electronic, and mechanical properties of D0c Zn3Ru structures, suggesting their suitability for engineering novel alloys in high-temperature structural applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
High-temperature structural materials have garnered significant attention due to their role in advancing industrial applications [1, 2]. Currently, Nickel-based super-alloys (NBSAs) are the predominant materials employed in the hot segments of turbine engines, primarily because of their exceptional high-temperature, physical and mechanical properties [3, 4]. However, the application of NBSAs is constrained in application in the next generation of turbine engines, primarily due to the limitation on the low melting point temperature of Ni (1543 °C). In this context, various metallic systems, including Refractory metals (RMs) such as Ti, Mg, Pt, Ru, and Cr-based alloys and the platinum group of metals (PGMs) have been proposed as viable alternatives to overcome the melting point temperature limitations associated with NBSAs.
Refractory metals (RM) based on Mo, Nb, Ta, and W suffer substantial oxidation challenges in air at elevated temperatures (500 °C), commonly referred to as pesting [5]. In addition, the specific strength of titanium alloys tends to deteriorate with increasing temperature, while the high-temperature application of magnesium alloys is limited by their constrained mechanical properties, including deficient strength, internal fatigue, and creep resistance [6]. Recent investigations have delved into Mg–X (X = La, Nd, and Sm) intermetallic alloys across various crystallographic phases such as D0c, A15, and L12, utilizing an ab initio density functional theory calculation [7, 8]. Notably, the heats of formation data indicate the thermodynamic stability in all phases. Additionally, these alloys demonstrate mechanical stability, except for the L12 and A15 phases.
The Platinum group metals (PGMs), such as Pt, Ru, Os, Rh, Pd, and Ir, for high-temperature structural applications has been investigated [9–12]. Despite their chemical properties resembling those of Nickel-based super-alloys (NBSAs) [13], some PGMs are inherently brittle and face challenges related to weight and cost. This limits their potential use in high-temperature applications. In a prior study by Chauke et al, [14] the phase stability of Pt3Al across various crystallographic phases, including L12, D0c' (tI16-Ir3Si), and tP16 (Pt3Ga) indicated that that Pt3Al in the tP16 phase exhibits the highest thermodynamic stability. Meanwhile, Mishima et al [15, 16] observed a phase transformation in Pt3Al, shifting from L12 to D0c at 400 °C–430 °C and from L12 to D0c' at 130 °C [17, 18]. Raub et al [19] and Greenfield et al [20] confirmed the presence of three intermetallic compounds—Cr4Ru, Cr3Ru, and Cr2Ru (σ phase)—in the Cr-Ru binary system, as well as the terminal solution phases BCC(Cr) and HCP(Ru), using metallography, x-ray diffraction (XRD), and microhardness assessment. Tibane et al [21] further investigated the phase stability of Cr3A and A3Cr (A = Pt, Ru) in various crystallographic phases such as D0c, D0c' tP16, A15, B2, L12 revealing that PtCr3 in the L12 phase represents the ground-state configuration. Other structures were deemed unstable but could potentially be stabilized through doping. Subsequently, many researchers have focused on investigating Cr-Ru alloys and modifying them with various 3d-transition metals [22–25]. The use of 3d-transition metal in Ru based alloys stems from their cost-effectiveness [26] and the magnetic characteristics, particularly notable in Cr, Mn, and Fe. Additionally, these metals generally exhibit higher melting points in comparison to main group metals [27] and possess relatively low densities [28]. Elements such as Ni, Co, Mn, Cu, Ti, Fe, and V have been noted for their ability to enhance the dehydrogenation properties of Magnesium hydride [29], enhance thermodynamic stability [23], and augment room temperature ductility in various materials [30]. The bonding behavior in transition metals is shaped by the contribution of both outermost and inner shell electrons [27]. When 3d-transition metals form alloys with other metals or elements, they generate sturdy materials highly desired for a variety of applications, including high-temperature structural, catalytic, opto-electronic, and spintronics purposes [25, 26, 31].
In a study by Mnisi et al [23], it was observed that the stability of RuCr3 and CrRu3 alloys in the A15 phase can be enhanced by introducing manganese under different concentrations. Furthermore, an examination of A15 Ru-based 3d transition metals revealed thermodynamic stability and high magnetic moment in the Mn3Ru whilst, other alloys were found to be structurally unstable, attributed to positive heats of formation [32]. Considering the diverse studies which have been carried out for Cr3Ru in various phases such as L12, A15, tP16, D0c, and D0c', the focus of our current study is on exploring the Ru-based alloys 3d-transition metals intermetallic alloys in the D0c crystallographic phase X3Ru (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn). Specifically, this paper explores the structural, electronic, and elastic properties these intermetallic alloys, offering valuable insights into the design of high-temperature structural materials. Notably, there is limited literature providing data on the physical properties of these structural compositions. Our results reveal that all D0c X3Ru systems exhibit metallic behavior, with high melting temperatures exceeding 700 K. Among these intermetallic alloys, Sc3Ru, Ti3Ru, V3Ru, Mn3Ru, and Zn3Ru stand out as thermodynamically stable, with elevated lattice constants and low densities. Conversely, all D0c X3Ru intermetallic alloys demonstrate mechanical stability, except for Sc3Ru, Ti3Ru, and Fe3Ru. Additionally, these compounds exhibit ductility, except for Ti3Ru, V3Ru, and Cr3Ru, aligning with the findings from Poisson's ratio analysis.
2. Computational details
Ab-initio pseudopotential Density Functional Theory calculations (DFT) were employed to assess the properties of tetragonal D0c X3Ru intermetallic alloys, using the CASTEP code [33]. The electronic exchange–correlation was described using the generalized gradient approximation (GGA) [34], and the Vanderbilt ultra-soft pseudopotential [35–38] was used to represent electronic valence-core interactions.
The ground state electronic and magnetic properties of the D0c X3Ru alloys were modeled using their respective unit cells, as illustrated in figure 1. Plane wave basis sets were utilized to represent electronic wave functions, with a converged cut-off energy of 800 eV., while optimized Monkhorst–Pack k-grid sampling of 15 × 15 × 10 was used for spin and geometry optimization [39]. To ensure the accuracy of the results, a convergence criteria for energy, force displacement, and stress were set at 5.0 × 10−6 eV per atom, 0.01 eV Å, and 0.02 GPa, while a temperature smearing width of 0.001 eV was set to ensure accuracy of the calculated magnetic moments. Similar convergence criteria were applied in the calculation of electronic and mechanical properties. For phonon calculations, a finite displacement method was employed [40–43] using a larger supercells of 4 × 4 × 4, a cut-off radius of 5.0 Å, and a k-grid sampling of 5 × 5 × 3.
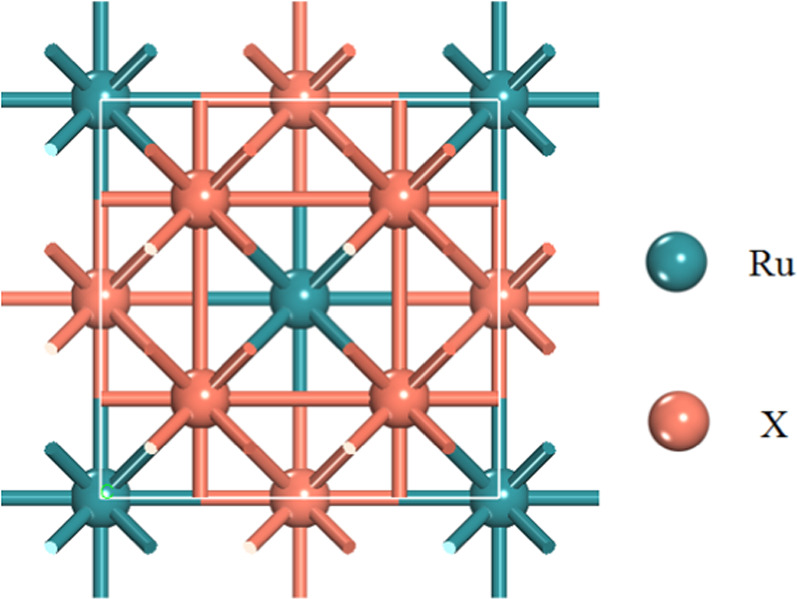
Figure 1. Crystal structure of tetragonal D0c X3Ru alloy. Blue ball represents Ru atom, while red balls represent transition metal atoms X (Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn).
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Heats of formation and structural stability
The crystal structures of X3Ru alloys (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn) belong to the body centered tetragonal D0c phase with the I4/mcm space group. Within the unit cell, the Ru atoms are positioned at the Wyckoff site 4a (0, 0, 0.25), while X atoms are located at 4b (0, 0.5, 0.25) and 8 h (0.25, 0.75, 0) positions, as illustrated in figure 1.
To determine the equilibrium ground state properties of X3Ru alloys, structural optimization was executed under zero temperature-pressure conditions. The results for the equilibrium lattice constants, volume, heats of formation, magnetic moments, and density are presented in table 1. Notably, an observed trend in X3Ru (X = Sc-Cu) reveals a consistent decrease in calculated lattice parameters (hence volume) across the 3d series, aligning with the trends in atomic radii of the 3d elements across the series. Table 2 shows the calculated and experimental lattice parameters for all 3d-transition metal elements such as Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu and Zn. We note that the calculated and experimental data agree.
Table 1. Equilibrium lattice constants a and c, equilibrium volume V0, heats of formation, magnetic moments, density and X-Ru bond-lengths of X3Ru, calculated at zero pressure.
| Phase | a (Å) | c (Å) | V0 (Å3) | ΔHf (eV/atom) | Magnetic moments (μB/atom) | Density (g/cm3) | X-Ru Bond length (Å) |
|---|---|---|---|---|---|---|---|
| Sc3Ru | 6.24 | 8.86 | 344.815 | −0.08 | 0.72 | 4.54 | 3.126 |
| Ti3Ru | 5.82 | 8.18 | 277.231 | −0.37 | 1.35 | 5.86 | 2.901 |
| V3Ru | 5.74 | 8.12 | 267.209 | −0.27 | 2.19 | 6.31 | 2.869 |
| Cr3Ru | 5.86 | 8.36 | 287.662 | 0.13 | 2.97 | 5.94 | 2.945 |
| Cr3Rutheo[21] | 5.20 | 0.24 | |||||
| Mn3Ru | 5.75 | 8.14 | 268.974 | −1.61 | 3.25 | 6.57 | 2.876 |
| Fe3Ru | 5.04 | 8.31 | 211.394 | 0.11 | 2.52 | 8.43 | 2.737 |
| Co3Ru | 5.20 | 7.33 | 198.305 | 0.10 | 1.82 | 9.31 | 2.597 |
| Ni3Ru | 5.14 | 7.28 | 192.870 | 0.92 | 1.08 | 9.55 | 2.574 |
| Cu3Ru | 5.21 | 7.37 | 200.393 | 0.31 | 0.00 | 9.67 | 2.607 |
| Zn3Ru | 5.36 | 7.57 | 217.358 | −0.22 | 0.00 | 9.08 | 2.678 |
Table 2. The calculated and experimental lattice parameters for Sc, Ti,V,Cr, Mn, Fe, Co, Ni, Cu and Zn elements.
| Calculated Å | Experimental Å | Previous theoretical Å | |
|---|---|---|---|
| Sc | 3.41 | 3.31 [44] | |
| Ti | 2.99 | 2.95 [44] | |
| V | 3.03 | 3.03 [44] | |
| Cr | 2.845 | 2.88 [45] | 2.851 [21] |
| Mn | 8.91 | 8.91 [44] | |
| Fe | 2.88 | 2.87 [44] | |
| Co | 2.51 | 2.51 [44] | |
| Ni | 3.53 | 3.52 [44] | |
| Cu | 3.64 | 3.61 [44] | |
| Zn | 2.63 | ||
| Ru | 2.71 | 2.71 [44] | 2.706 [21] |
| A15-Cr3Ru | 4.623 | 4.637 [24] | 4.631 [21] |
The heat of formation (ΔHf ) for an alloy is defined as the energy required to either form or break chemical bonds and is calculated as:
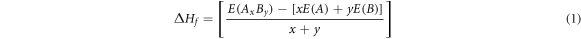
where, E(Ax By ), E(A), and E(B) are the calculated equilibrium total energies of the alloy system AB and that of the individual elemental species A and B, with atomic concentrations x and y respectively.
A negative value for ΔHf indicates chemical stability, while a positive value of ΔHf indicates instability. Table 1 displays the calculated heat of formation for X3Ru alloys. The calculated heat of formation for Cr3Ru is observed to be comparable to the previous theoretical value. The slight discrepancy can be primarily attributed to the use of different exchange correlation functionals. In the previous study, the GGA functional was employed, whereas in this investigation, the GGA + U (U = 2.5 eV) was used. Notably, Sc3Ru, Ti3Ru, V3Ru, Mn3Ru, and Zn3Ru exhibit negative heat of formation, signifying thermodynamic stability. Therefore, these alloy systems can readily be synthesized experimentally under equilibrium conditions. Considering that the stability of intermetallic alloys is intertwined with their magnetic moments through orbital hybridization, we also computed the magnetic moments of X3Ru alloys. It was observed that all X3Ru alloys are magnetic except Cu3Ru and Zn3Ru. Additionally, it is noteworthy that most of the stable systems display significant magnetic moments, with Mn3Ru having the highest magnetic moment.
The density of materials is a vital tool used to characterize its use in lightweight applications such as in aerospace, and is calculated as:
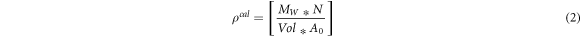
where  represents the volume of a unit cell,
represents the volume of a unit cell,  is the average molecular weight of the elements in the unit cell,
is the average molecular weight of the elements in the unit cell,  is the total number of atoms and
is the total number of atoms and  is the Avogadro's number (6.022 × 1023). Table 1 gives the calculated densities of D0c X3Ru alloys. Importantly, it is observed that the densities of the most stable alloys, namely Sc3Ru (4.54 g/cm3), Ti3Ru (5.86 g/cm3), V3Ru (6.31 g cm−3), and Mn3Ru (6.57 g cm−3), are comparable to that of L12-Ni3Al (6.14 g cm−3) [12] commonly employed in the aerospace industry. Therefore, these structures are promising candidates for high-temperature lightweight structural applications.
is the Avogadro's number (6.022 × 1023). Table 1 gives the calculated densities of D0c X3Ru alloys. Importantly, it is observed that the densities of the most stable alloys, namely Sc3Ru (4.54 g/cm3), Ti3Ru (5.86 g/cm3), V3Ru (6.31 g cm−3), and Mn3Ru (6.57 g cm−3), are comparable to that of L12-Ni3Al (6.14 g cm−3) [12] commonly employed in the aerospace industry. Therefore, these structures are promising candidates for high-temperature lightweight structural applications.
Research on D0c X3Ru alloys is relatively new and as such there is limited data of these materials systems in literature. Our calculated lattice constant of Cr3Ru—which is the prototypical alloy in this class of materials agrees with literature. To further validate our theoretical model, our calculated lattice parameters for the individual elements (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu) are agreement with experimental values (table 2).
3.2. Electronic and magnetic properties
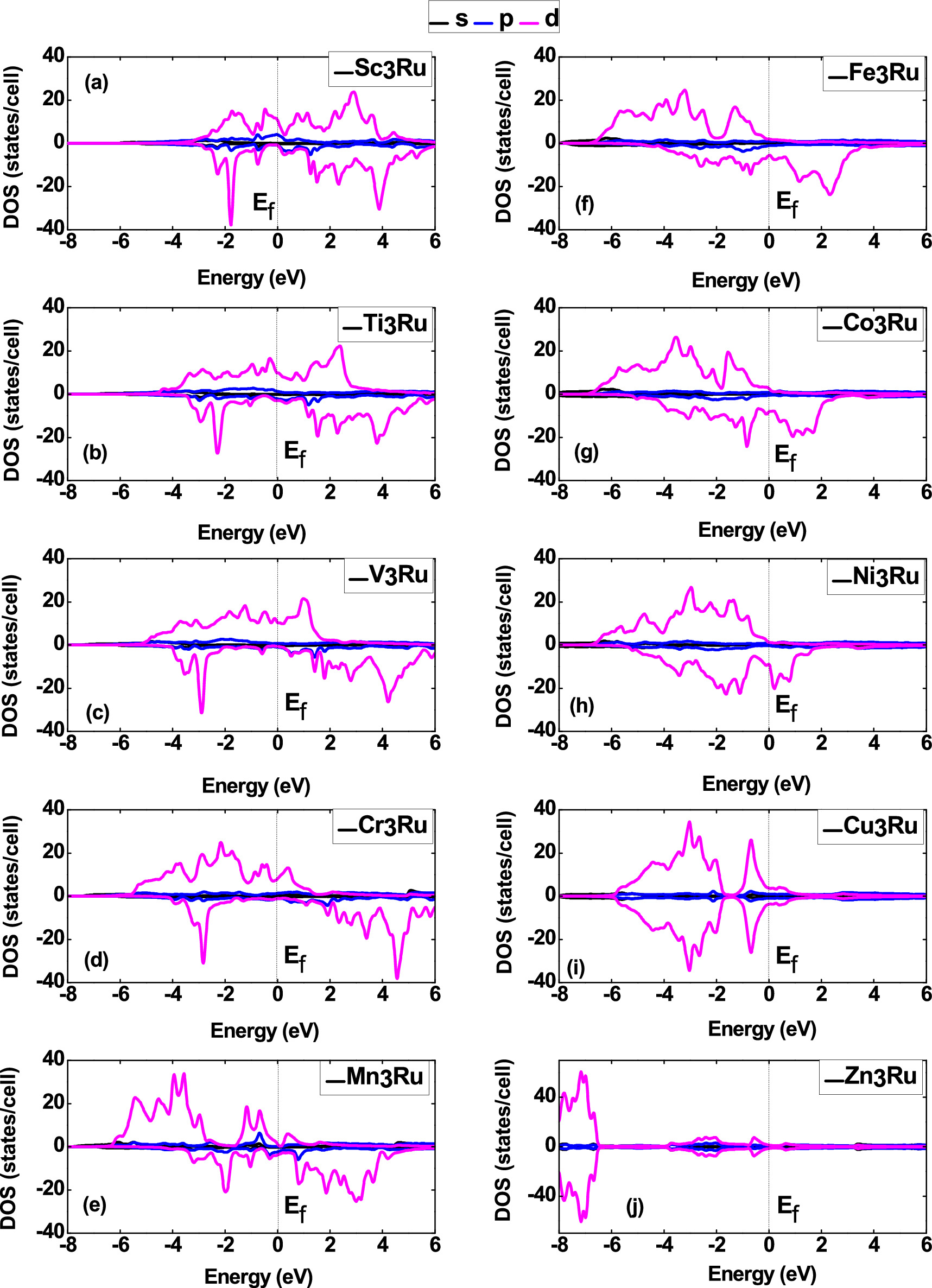
Understanding the density of states (DOS) is crucial for clarifying the link between the stability of a material and its magnetic properties [46]. The partial density of states (PDOS) for X3Ru is shown in figure 2, showing the contribution arising from the s, p and d orbitals in the unit cell. Significantly, there is an electronic overlap between the valence and conduction bands near the Fermi energy level, suggesting metallic conductivity in these alloys. In Cu3Ru and Zn3Ru, the spin up and spin down bands are symmetric, hence zero spin polarization. This symmetry leads to the elimination of the magnetic moment linked to electronic spin, explaining the absence or minimal calculated magnetic moments (table 1). Conversely, the density of states in Sc3Ru, Ti3Ru, V3Ru, Cr3Ru, Mn3Ru, Fe3Ru, and Co3Ru indicates spin polarization, confirming the existence of magnetic moments in these systems. The existence of magnetic moments in these alloys makes them potentially viable for applications in spintronics and spin injection. These findings align with our earlier research in similar material systems [32, 47–49].
Figure 2. Calculated Partial density of states of X3Ru (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu and Zn) alloys. The zero indicates the Fermi energy (0 eV). The vertical scale for all X3Ru alloys is the same for comparison's sake, except in Zn3Ru.
Download figure:
Standard image High-resolution imageNotably, around the Fermi energy, a prominent feature is the presence of a valley referred to as the pseudo-gap in both the spin-up and spin-down bands, which indicates covalent bonding [50, 51]. The existence of the pseudo-gap arises from strong hybridization in X-3d and Ru-3d states, effectively separating the bonding states from the anti-bonding states.
3.3. Mechanical stability
Mechanical stability serves as a metric to gauge a material's strength and is employed for characterizing the structural stability and deformation of a system under external load [52]. This stability is defined in terms of elastic constants Cij , Bulk Modulus (B), Shear Modulus (G), and Young's modulus (E), providing insights into a material's hardness and ductility. For tetragonal crystals, mechanical stability criteria at zero pressure are defined by the following set of equations [53];
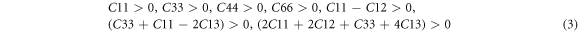
In tetragonal X3Ru (X = Sc-Zn) alloys, the six independent elastic constants C11 , C33 , C12 , C44 , C13 , and C66 are outlined in table 3. It is noteworthy that the elastic constants of V3Ru, Ni3Ru, Co3Ru, Cu3Ru, Mn3Ru, Cr3Ru, and Zn3Ru meet the Bohr mechanical stability criteria (Cij > 0), signifying their mechanical stability. In contrast, Sc3Ru, Ti3Ru, and Fe3Ru structures have C44 and C66 values below zero, indicating mechanical instability.
Table 3. Calculated independent elastic constants (Cij) in GPa and melting temperature (Tm) in Kelvin for X3Ru (X = Sc, Ti, V, Fe, Co, Ni, Cu and Zn) alloys.
| Phase | C11 | C12 | C13 | C33 | C44 | C66 | Tm ±300 K |
|---|---|---|---|---|---|---|---|
| Sc3Ru | 95.50 | 34.04 | 68.84 | 61.12 | 34.35 | −6.53 | 732.18 |
| Ti3Ru | 110.70 | 85.74 | 104.34 | 68.60 | −5.09 | −8.45 | 789.00 |
| V3Ru | 200.46 | 29.87 | 62.43 | 146.96 | 91.71 | 52.81 | 1175.82 |
| Cr3Ru | 78.43 | −36.32 | 16.21 | 55.37 | 55.27 | 0.81 | 1309.04 |
| Mn3Ru | 174.56 | 17.00 | 76.63 | 130.40 | 77.66 | 26.59 | 1073.28 |
| Fe3Ru | 352.98 | 39.70 | 113.18 | 272.33 | 37.41 | −51.85 | 1821.44 |
| Co3Ru | 339.63 | 82.31 | 181.13 | 229.83 | 111.85 | 24.84 | 1717.64 |
| Ni3Ru | 326.33 | 110.64 | 167.05 | 282.16 | 112.89 | 56.06 | 1756.23 |
| Cu3Ru | 258.39 | 90.88 | 170.72 | 195.35 | 93.85 | 28.86 | 1422.20 |
| Zn3Ru | 256.25 | 72.76 | 119.52 | 218.79 | 92.20 | 37.19 | 1450.94 |
The melting temperature ( ) of a material depends on its mechanical properties, and it follows a linear relationship with its elastic constants [54–57]. For tetragonal structures, the melting point is given by equation (4):
) of a material depends on its mechanical properties, and it follows a linear relationship with its elastic constants [54–57]. For tetragonal structures, the melting point is given by equation (4):
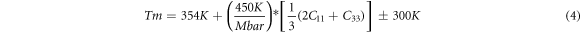
In table 3, we noticed a rising pattern in melting temperatures from Sc to Zn alloys, with Fe3Ru exhibiting the highest melting temperature. Moreover, the melting temperatures of Fe3Ru, Co3Ru, and Ni3Ru exceed those of the presently employed L12 Ni3Al (1691 K [12] and 1668 K [58]). As a result, these particular alloys present themselves as promising candidates for high-temperature structural conditions.
The Bulk Modulus (B) is a measure for a material's resistance to compression, and its magnitude is influenced by the crystal structure of the material. Materials with high compressibility exhibit large values of bulk modulus, while low bulk modulus values indicate materials with low compressibility. In the context of tetragonal structures, the expression for B is:
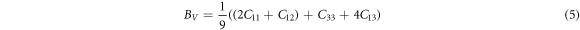
and the Reuss bounds are:
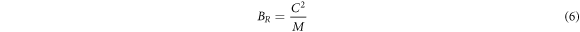
where,
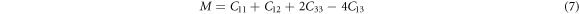
with BR , B = BH and BV being the Bulk modulus for Reuss, Voigt and Hill approximations [59]. The shear modulus (G) of a material describes its response to shear stress and is a measure of a material's stiffness. For tetragonal structures, G is expressed as
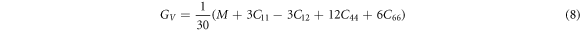
where, 
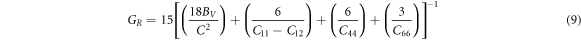
where, 
where the GR and GV are the Reuss and Voigt bounds [60], Young's modulus and Poison's ratio ʋ are independent of the type of a material's crystal structure and are given by:
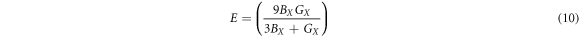
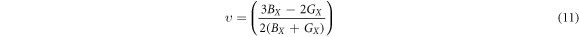
where, X = Voigt, Reuss and Hill approximations.
From the calculated elastic constants provided in table 3, the bulk modulus (B), shear modulus (G), and Young's modulus (E) are computed and presented in table 4. Across all the X3Ru alloys, it is noteworthy that BH,R consistently surpasses GH,R, indicating that the principal parameter governing the stability of base-centered tetragonal X3Ru alloys is the shear modulus [61]. On the other hand, Young's modulus characterizes a material's strain response to uniaxial stress, reflecting its stiffness. Higher values of Young's modulus are associated with stiffer materials, whereas lower values indicate less stiffness. Table 3 reveals that Ni3Ru stands out as the stiffest, while Ti3Ru is the least stiff.
Table 4. Calculated bulk modulus B (GPa), shear modulus (GPa), Young's modulus E (GPa), a ratio of bulk to shear modulus (BH/GH), Poisson's ratio (υ) and Vickers hardness (HV) of X3Ru (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu and Zn) alloys at zero pressure. V, R and H are the Voigt, Reuss and Hill approximations. UND = undefined.
| Phase | BV | GV | BR | GR | BH | GH | EH | BH/GH | υ | HV |
|---|---|---|---|---|---|---|---|---|---|---|
| Sc3Ru | 66.17 | 17.80 | 66.17 | −15.78 | 66.17 | 1.00 | 2.98 | 66.17 | 0.49 | −3.00 |
| Ti3Ru | 97.65 | −4.02 | 99.11 | −9.96 | 98.38 | −6.99 | −21.48 | −14.07 | 0.54 | UND |
| V3Ru | 95.26 | 73.46 | 94.91 | 67.20 | 95.08 | 70.33 | 169.26 | 1.35 | 0.20 | 13.92 |
| Cr3Ru | 22.71 | 36.68 | 20.52 | 3.68 | 21.62 | 20.18 | 46.17 | 1.07 | 0.14 | 7.70 |
| Mn3Ru | 91.16 | 57.00 | 90.75 | 42.60 | 90.93 | 49.80 | 126.34 | 1.83 | 0.27 | 6.73 |
| Fe3Ru | 167.82 | 52.08 | 167.80 | 94.45 | 167.81 | 73.26 | 191.86 | 2.29 | 0.31 | 6.35 |
| Co3Ru | 199.80 | 80.68 | 199.63 | 48.02 | 199.72 | 64.35 | 174.33 | 3.10 | 0.35 | 3.07 |
| Ni3Ru | 202.70 | 89.04 | 202.60 | 79.56 | 202.65 | 84.30 | 222.10 | 2.40 | 0.32 | 6.59 |
| Cu3Ru | 175.20 | 61.97 | 174.10 | 28.80 | 174.65 | 45.38 | 125.29 | 3.89 | 0.38 | 0.85 |
| Zn3Ru | 150.54 | 72.29 | 150.48 | 62.28 | 150.51 | 67.28 | 175.67 | 2.24 | 0.31 | 6.15 |
We find that the elastic moduli across the 3d-series (X) is generally lower in the early 3d-elements and higher in the middle 3d-element, with Fe3Ru, Co3Ru and Ni3Ru having the largest B and G values. Interestingly, we therefore conclude that, high elastic moduli in X3Ru alloys correspond to the high melting temperature (figure 3). Further, the bulk, shear, young's moduli have a monotonic relationship with the melting temperature. Therefore, increased stiffness, hardness, and compression resistance in these structures explains the higher melting temperatures correspond. These findings align with the trends previously observed by Popoola et al [12].
Figure 3. Trends in melting temperature (MT in °C), bulk modulus (B), Shear modulus and Young's modulus (E) in GPa across the 3d series in X3Ru alloys (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu and Zn).
Download figure:
Standard image High-resolution imageApplying Pugh's criterion [62], the ratio of bulk to shear modulus (BH/GH) is employed to predict the ductility/brittleness of a material, with the critical value set at 1.75. Ductile behavior is predicted if BH/GH is greater than 1.75; otherwise, the material is deemed brittle. Notably, Sc3Ru Mn3Ru, Fe3Ru, Co3Ru, Ni3Ru, Cu3Ru, and Zn3Ru exhibit ductile behavior, while Ti3Ru, V3Ru, and Cr3Ru display brittleness. Additionally, the ductility/brittleness of materials can be assessed using Poisson's ratio, with the threshold value set at 0.33 [63]. Employing the Poisson's ratio criteria, it is observed that all X3Ru alloys are ductile except V3Ru and Cr3Ru, aligning with the results from Pugh's criterion, as shown in the figure 4.
Figure 4. Calculated trends in the ratio of the bulk to shear modulus and Poisson's ratio across the 3d series in X3Ru X3Ru alloys (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu and Zn).
Download figure:
Standard image High-resolution imageIn evaluating the crystal's stability against shear deformation, Poisson's ratio has been considered [64, 65]. A higher Poisson's ratio indicates better plasticity in crystals, and it also serves as a characterization of the bonding forces within crystals [66]. The central forces in a solid typically fall within the range of 0.25 ≤ υ ≤ 0.5. The computed Poisson's ratios (υ) for X3Ru range from 0.14 to 0.54. All the structures exhibit a Poisson's ratio within the range of 0.25 ≤ υ ≤ 0.5, confirming that the interatomic forces in these structures are central, except for Ti3Ru, V3Ru and Cr3Ru.
Hardness is a critical attribute in high-temperature environments, particularly in aerospace applications. It defines a material's resistance to localized plastic deformation caused by either mechanical indentation or abrasion. Hardness, while not a fundamental property, relies on tensile strength, yield strength, and the modulus of elasticity. It is determined through the Vickers hardness equation (12) [67]
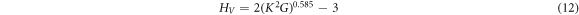
where K represents the ratio of shear modulus to bulk modulus (GH/BH), and H denotes the Hill approximation. The calculated hardness values for tetragonal X3Ru structures are presented in table 3. It is evident that V3Ru exhibits the highest hardness, while Sc3Ru has the lowest. Notably, the hardness for Ti3Ru is undefined due to the negative square root in GH/BH, indicating instability associated with a phase change in this structure. This type of instability is also observed in ferro-elastic phase transformations [68], as explained by Landau theory [69], where two local minima form in a strain energy function.
3.4. Elastic anisotropy
Understanding the anisotropic behavior of a material is crucial in engineering science and crystal physics, as it is closely related to micro-cracks in materials. Calculating elastic anisotropy provides valuable information about a material, including details about micro-cracks, phase transformation, precipitation, and dislocation dynamics [70]. For tetragonal alloys, elastic anisotropy can be expressed through three elastic factors: shear anisotropy factors (such as A1 , A2 and A3 ) for different crystallographic planes, the universal anisotropic index (AU ), and the percentage in compression (AB ) anisotropy as given below:
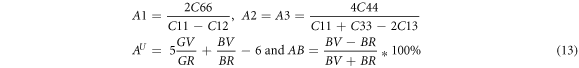
where A1 , A2 and A3 represent the shear anisotropic factors for the (001), (010) and (100) shear planes. In the case of locally isotropic structural materials, the shear anisotropy factors A1 , A2 and A3 are expected to be equal to one. Additionally, the universal anisotropic index AU should be zero in such materials. Any deviations from one or zero, indicates the degree of elastic anisotropy [71, 72]. The BV , BR , GV and GR represents the Voigt and Reuss approximation for bulk modulus (B) and shear modulus (G). The maximum value of (AB ) is 100%, which corresponds to maximum anisotropy [73, 74] while the minimum is zero which corresponds to an isotropic material.
Table 5 summarizes the calculated values of A1, A2, AU andAB in the X3Ru alloys. It is evident that A1 values in the X3Ru alloys are smaller than one, indicating elastically anisotropic structures. On the other hand, the A2 values are larger than one (except in Ti3Ru and Fe3Ru) suggesting that they are slightly isotropic. Regarding AU , it is observed that V3Ru and Ni3Ru and Zn3Ru exhibit a small degree of universal elastic isotropic behavior as they are close to unity. The (AB ) results clearly indicate that X3Ru (X = V, Mn, Fe, Co, Ni, Cu, and Zn) structures are close to zero, suggesting they are slightly isotropic, while Sc3Ru (0.0) is isotropic, indicating that these structures are predicted to have nearly identical values of AB in all x, y and z directions, attributing to external forces.
Table 5. The shear anisotropic factors (A1, A2), universal elastic anisotropy index (AU) and the anisotropy percentages (AB) of X3Ru3 (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu and Zn) alloys under zero pressure.
| Phase | A1 | A2 | AU | AB |
|---|---|---|---|---|
| Sc3Ru | −0.21 | 7.25 | −10.64 | 0 |
| Ti3Ru | −0.68 | 0.69 | −2.997 | −0.74 |
| V3Ru | 0.62 | 1.65 | 0.47 | 0.18 |
| Cr3Ru | 0.01 | 2.18 | 44.98 | 5.07 |
| Mn3Ru | 0.34 | 2.05 | 1.69 | 0.23 |
| Fe3Ru | −0.33 | 0.38 | −2.24 | 0.01 |
| Co3Ru | 0.19 | 2.16 | 3.40 | 0.04 |
| Ni3Ru | 0.52 | 1.65 | 0.60 | 0.02 |
| Cu3Ru | 0.35 | 3.34 | 5.77 | 0.31 |
| Zn3Ru | 0.41 | 1.56 | 0.62 | 0.02 |
3.5. Phonon dispersion
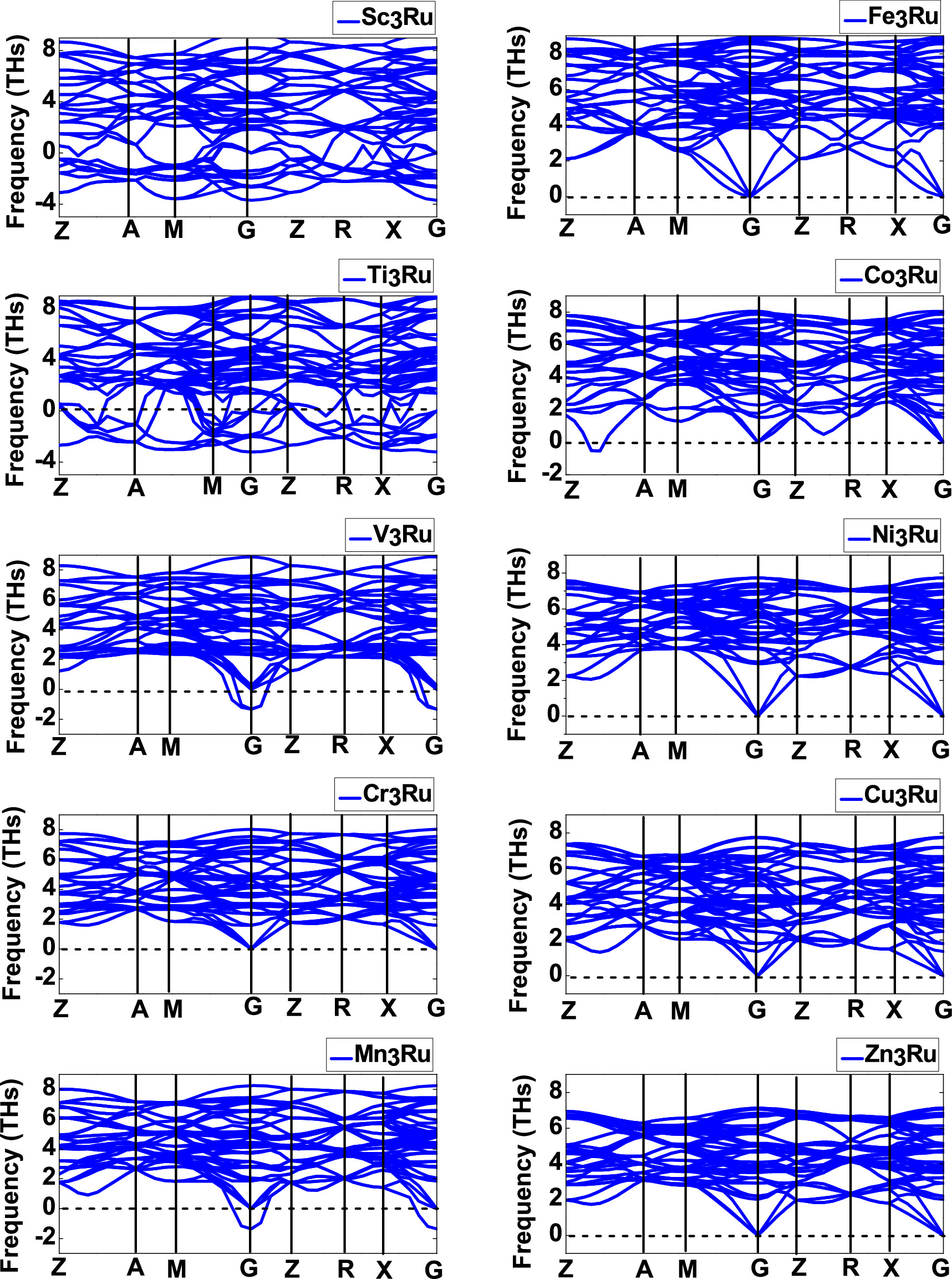
Phonons play a vital role in the dynamical behavior and thermal conductivities, which are critical aspects in the research advancements of novel materials. The phonon dispersion curves provide insights into dynamic stability or instability of a material by showcasing positive (real branches) and negative (imaginary branches) phonon frequencies. Positive phonon frequency modes signify dynamic stability in a material, while negative frequency modes indicate dynamic instability in a compound [75, 76]. Figure 5 shows the dispersion curves of D0c X3Ru (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn) at plotted along the highest symmetry k-points of the Brillouin zone. The phonon dispersion curves of Cr3Ru, Fe3Ru, Ni3Ru, Cu3Ru, and Zn3Ru are dynamically stable due to the absence of imaginary frequencies. Conversely, the phonon dispersion plots of Ti3Ru, V3Ru, Co3Ru, and Mn3Ru exhibit negative phonon modes, indicating dynamic instability, which can be drawback in the application of these materials where dynamic stability is critical.
In summary, by considering the thermodynamic, mechanical and dynamic stability of the X3Ru alloys presented in this study, our findings suggest that: (1) D0c X3Ru (X = Sc, Ti, V, Cr, Mn, and Zn) alloys exhibit negative heat of formation, hence are thermodynamical stable (2) X3Ru (X = V, Mn, Co, Ni, Cu, and Zn) alloys are mechanically stable, with Co3Ru and Ni3Ru exhibiting elevated melting temperatures compared to the currently utilized alloy L12 Ni3Al (1691 °C) [12] (3) X3Ru (X = Cr, Fe, Ni, Cu, and Zn) are dynamically stable. Therefore, Zn3Ru meets all three stability criteria, which could give a slight advantage for structural applications over the other systems examined in this work.
Figure 5. Dispersion curves for D0c X3Ru (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu and Zn) compounds at high symmetric points in the Brillouin zone.
Download figure:
Standard image High-resolution image4. Conclusion
In conclusion, we employed Density Functional Theory to calculate the electronic, magnetic, elastic, and thermodynamic properties of binary D0c X3Ru (X = Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn) tetragonal alloys. Our investigation reveals that D0c X3Ru (X = Sc, Ti, V, Cr, Mn, and Zn) alloys exhibit negative heat of formation, indicating structural stability. Notably, Ti3Ru, V3Ru, Cr3Ru, Mn3Ru, Fe3Ru, Co3Ru, and Ni3Ru compounds display high magnetism attributed to strong spin polarization. Mechanical property analysis demonstrate that X3Ru (X = V, Mn, Co, Ni, Cu, and Zn) alloys are mechanically stable. Additionally, the mechanically stable structures of Co3Ru and Ni3Ru exhibit elevated melting temperatures compared to the currently utilized alloy L12 Ni3Al (1691 °C) [12], positioning them as potential candidates for high-temperature structural applications. Phonon calculations indicated that Cr3Ru, Fe3Ru, Ni3Ru, Cu3Ru, and Zn3Ru are dynamically stable. These results provide a comprehensive overview of the stability, electronic, and mechanical properties of D0c Zn3Ru structures, suggesting their suitability for engineering novel alloys in high-temperature structural applications.
Acknowledgments
This work received support from the National Research Foundation (NRF) under grant number 121479 and the Grow Your Own Timber (GYOT) Project at UNISA. All DFT calculations were conducted at the University of South Africa, utilizing high-performance computing (HPC) resources.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
CRediT authorship contribution statement
B O Mnisi: Conceptualization, Methodology, Validation, Investigation, Writing—original draft, Project administration. E M Benecha: Writing—review & editing, Resources, Supervision. M M Tibane: Writing—review & editing, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.