- 1Department of Oncology, Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, NY, United States
- 2Center for Observational and Real-World Evidence, Merck & Co., Inc., Rahway, NJ, United States
- 3Clinical Research, Merck & Co., Inc., Rahway, NJ, United States
Background: Front-line therapy with an EGFR tyrosine kinase inhibitor (TKI) is the standard of care for treating patients with advanced nonsquamous NSCLC with the common sensitizing EGFR exon 19 deletion and exon 21 L858R point mutations. However, EGFR TKI resistance inevitably develops. The optimal subsequent therapy remains to be identified, although platinum-containing chemotherapy regimens are often administered. Our objectives were to describe baseline characteristics, survival, and subsequent treatment patterns for patients with advanced nonsquamous NSCLC with EGFR exon 19 deletion or L858R mutation who received a platinum-based combination regimen after front-line EGFR TKI therapy.
Methods: This retrospective study used a nationwide electronic health record-derived deidentified database to select adult patients with advanced nonsquamous NSCLC, evidence of EGFR exon 19 deletion or L858R mutation, and ECOG performance status of 0-2 who initiated platinum-containing chemotherapy, with or without concomitant immunotherapy, from 1-January-2011 to 30-June-2020 following receipt of any EGFR TKI as first-line therapy or, alternatively, a first- or second-generation EGFR TKI (erlotinib, afatinib, gefitinib, dacomitinib) as first-line therapy followed by the third-generation EGFR TKI osimertinib as second-line therapy. Data cut-off was 30-June-2022. The Kaplan-Meier method was used to estimate overall survival (OS) after initiation of pemetrexed-platinum (n=119) or any platinum-based combination regimen (platinum cohort; n=311).
Results: The two cohorts included two-thirds women (65%-66%) and 57%-58% nonsmokers; median ages were 66 and 65 years in pemetrexed-platinum and platinum cohorts, respectively. Median OS was 10.3 months (95% CI, 8.1-13.9) from pemetrexed-platinum initiation and 12.4 months (95% CI, 10.2-15.2) from platinum initiation; 12-month survival rates were 48% and 51%, respectively; 260 patients (84%) had died by the end of the study.
Conclusion: The suboptimal survival outcomes recorded in this study demonstrate the unmet need to identify more effective subsequent treatment regimens for patients with EGFR-mutated advanced nonsquamous NSCLC after EGFR TKI resistance develops.
1 Introduction
Knowledge of the molecular biology of non-small cell lung cancer (NSCLC) has rapidly expanded in recent years, together with the development of therapies targeting specific oncogenic drivers (1). Mutations in the epidermal growth factor receptor (EGFR) gene are common in lung adenocarcinoma, with an estimated prevalence in lung adenocarcinoma of 23% (range, 3–42%) in the United States (US) and greater frequency in Asian than non-Asian patients, in women than men, and in never-smokers (vs. smokers) (2, 3). The most common sensitizing EGFR mutations are exon 19 deletions (ex19del) and exon 21 L858R point mutations (3–6).
Testing for EGFR mutations in the advanced NSCLC setting came into standard practice as early as 2011, when clinical trials were examining the place in therapy of the first-generation EGFR tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib (7). These agents, as well as the second-generation EGFR TKIs afatinib and dacomitinib and the third-generation EGFR TKI osimertinib have demonstrated superiority over chemotherapy for treating EGFR-mutated NSCLC as front-line therapy (6, 8). Osimertinib was originally approved for treating the EGFR T790M mutation in exon 20, the most common mutation conferring resistance to first- and second-generation EGFR TKIs (4, 6). Front-line therapy with osimertinib is current standard of care for patients with stage IV NSCLC with EGFR ex19del or L858R mutation and Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2 based on the FLAURA trial results demonstrating improved outcomes over first-generation agents (9). However, drug resistance typically develops also with osimertinib. Diverse resistance mechanisms to the EGFR TKIs include on-target alterations, bypass signaling pathway activation, and histological transformation (6, 10).
Identifying the optimal subsequent therapy for patients who experience disease progression after EGFR TKI therapy remains an active area of study (11–14). Clinical trial results suggest no benefit of adding immunotherapy to platinum-based therapies for treating TKI-resistant metastatic NSCLC (15–18), with the exception of a small subgroup analysis of patients with EGFR-mutated NSCLC and prior TKI exposure who received atezolizumab added to a bevacizumab-carboplatin-paclitaxel regimen (19), a regimen that nonetheless did not garner US regulatory approval. Moreover, recently reported interim analyses of the ORIENT-31 trial suggest benefits in progression-free survival for patients receiving sintilimab plus chemotherapy, with or without bevacizumab biosimilar IBI305, as compared with chemotherapy alone (20, 21). Current clinical guidelines list multiple regimens, most of them platinum-based, to consider after EGFR TKI resistance develops (9, 22, 23).
Data from real-world oncology practice can provide information to supplement clinical trial data (24, 25). Recent observational studies have examined first-line EGFR TKI treatment patterns and outcomes for patients with EGFR-mutated advanced NSCLC (26–28); however, information remains limited regarding the outcomes of platinum-based regimens administered after disease progression on EGFR TKI therapy in real-world settings (29). The aims of this study were to describe baseline characteristics, survival, and subsequent treatment patterns for patients with advanced nonsquamous NSCLC with EGFR ex19del or L858R mutation who received pemetrexed plus platinum or other platinum-based combination regimen as the next line of therapy after front-line EGFR TKI therapy at US oncology practices.
2 Methods
2.1 Data source and patients
The nationwide Flatiron Health database contains deidentified, electronic health record-derived patient-level data from oncology practices throughout the US, including approximately 280 cancer clinics (~800 sites of care) at the time of this study. The longitudinal, structured and unstructured data are curated via technology-enabled abstraction and include patient characteristics and lines of systemic anticancer therapy defined by oncologist-defined, rules-based methods, as previously described (30–32).
We studied patients in the Flatiron Health advanced NSCLC database, which includes patients with at least two recorded clinic visits and a pathologically confirmed diagnosis of advanced NSCLC (unresectable stage IIIB/IIIC, or stage IV) on or after 1 January 2011. Eligible patients were ≥18 years old with nonsquamous NSCLC and evidence of EGFR ex19del or L858R mutation. We selected those who initiated platinum-containing chemotherapy from 1 January 2011 to 30 June 2020 after having received EGFR TKI therapy.
Eligible patients had received any EGFR TKI as first-line therapy or, alternatively, a first- or second-generation EGFR TKI (erlotinib, afatinib, gefitinib, dacomitinib) as first-line therapy followed by osimertinib in second line. From these patients, we then selected those who received combination pemetrexed plus platinum (pemetrexed-platinum cohort) as subsequent therapy. Because patients with progression after an EGFR TKI can also receive other platinum combination therapies, we selected a second cohort that included all patients who received any platinum-based combination regimen, with or without concomitant immunotherapy (platinum cohort). Thus, the platinum cohort also included patients who received pemetrexed-platinum, and the two cohorts were not mutually exclusive. (Platinum was defined as carboplatin or cisplatin.)
Patients enrolled in a clinical trial or with ECOG PS of ≥3 were excluded. In addition, we excluded those who had no clinic visit ≤90 days after the advanced NSCLC diagnosis, as a potential indication of insufficient follow-up. The data cutoff date was 30 June 2022, allowing for a minimum follow-up period of 2 years from the time of initiating platinum-containing chemotherapy.
Institutional Review Board approval of the study protocol was obtained before conducting the study and included a waiver of informed consent for working with deidentified data. The deidentified data were subject to obligations to prevent reidentification during the analyses to protect patient confidentiality.
2.2 Outcomes and analyses
We summarized patient demographics and clinical characteristics at initiation of platinum-containing chemotherapy (defined as the “index date”) for the pemetrexed-platinum cohort and the platinum cohort. The baseline ECOG PS was identified as that recorded closest to the index date and within 30 days before to 30 days after the index date. In addition, we summarized the platinum-containing regimen types and the regimens administered in the next line of therapy (namely, third line or fourth line, depending whether osimertinib was administered in second line).
The Kaplan-Meier method was used to estimate median overall survival (OS) with 95% confidence intervals (CIs) and survival rates at 12 and 24 months; patients who were alive at data cutoff were censored. Dates of death were determined using a validated real-world mortality endpoint (33–35). We also conducted a sensitivity analysis to estimate OS for patients with ECOG PS of 0 or 1.
All patients who met eligibility criteria were included in descriptive and Kaplan-Meier survival analyses. No formal sample size or power calculations were conducted; and SAS software, version 9.4 (SAS Institute, Cary, NC) was used for the analyses.
3 Results
3.1 Patients and therapy
The deidentified database included 2975 patients with EGFR-mutated nonsquamous NSCLC who received a first-line EGFR TKI with or without second-line osimertinib (Figure 1). Of these 2975 patients, 320 (11%) had a record of then initiating a platinum-containing regimen from 2011 to mid-2020. After excluding 9 patients (3%) with ECOG PS of 3 or 4, we studied 119 patients in the pemetrexed-platinum cohort and 311 patients in the platinum cohort (Figure 1).
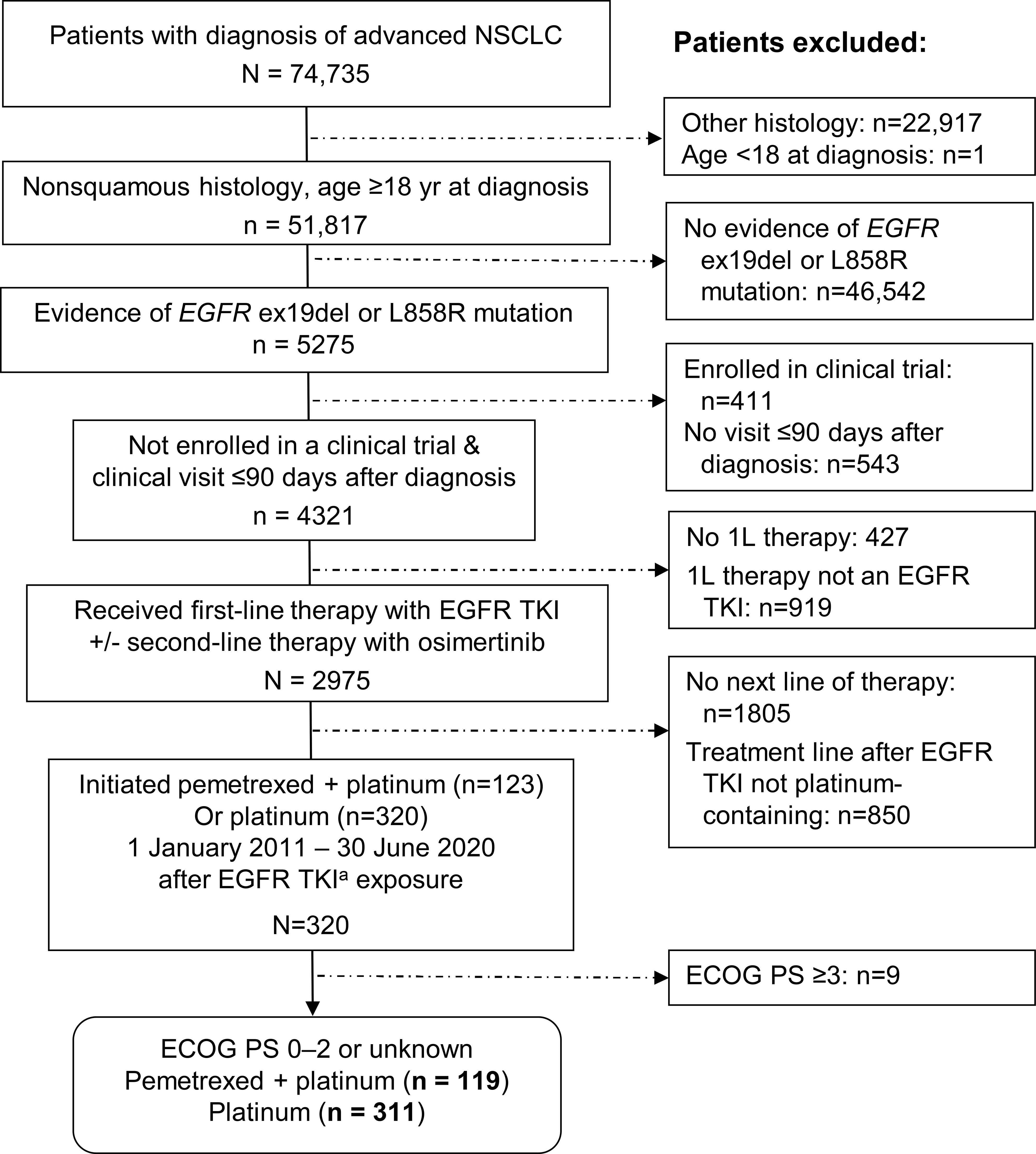
Figure 1 Selection of eligible patients from the deidentified database. aEGFR TKI exposure included any EGFR TKI as first-line therapy or first-/second-generation EGFR TKI in first line and third-generation EGFR TKI (osimertinib) in second line. 1L, 2L, 3L, first-, second-, third-line therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR ex19del, EGFR exon 19 deletion; TKI, tyrosine kinase inhibitor.
Baseline demographic characteristics of patients in the two cohorts were similar. Median ages were 66 and 65 years, including 20% and 19% aged ≥75 years and 65% and 66% women in the pemetrexed-platinum and platinum cohorts, respectively (Table 1). Approximately three-quarters of patients with known race were White, 6% to 8% were Black, while 18% in the pemetrexed-platinum and 15% in the platinum cohort were Asian. The majority of patients were treated at community oncology practices (78% and 82%, respectively).
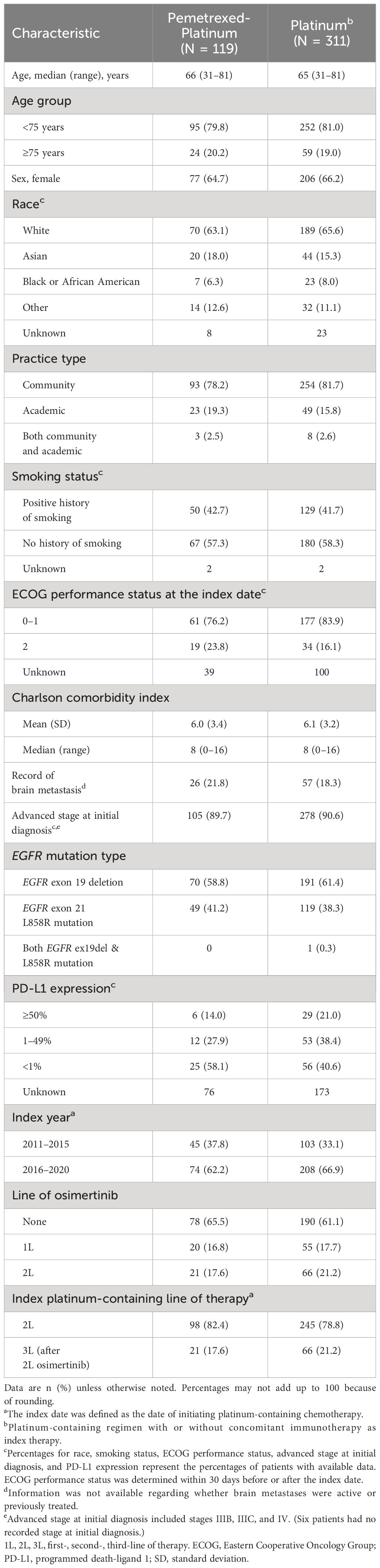
Table 1 Characteristics at the index date of patients with EGFR-mutated nonsquamous advanced NSCLC previously treated with an EGFR TKIa.
Most patients (90% and 91%, respectively) received an initial diagnosis of NSCLC at an advanced stage, while fewer than half of patients had a history of smoking (Table 1). In the pemetrexed-platinum and platinum cohorts, 22% and 18%, respectively, had a record of brain metastasis.
The EGFR mutations were identified most commonly via tissue samples (data not shown). One patient in the platinum cohort had a record of both EGFR ex19del and L858R mutation; all other patients had tumors with either EGFR ex19del or L858R mutation (Table 1).
The distribution of PD-L1 expression is summarized in Table 1 for the half or fewer patients in each cohort who had recorded results: namely, 36% and 44% of patients in the pemetrexed-platinum and platinum cohorts, respectively. The platinum-containing therapies are detailed in Supplementary Table 1. Overall, 87 of 311 patients (28%) received concomitant immunotherapy.
3.2 Outcomes
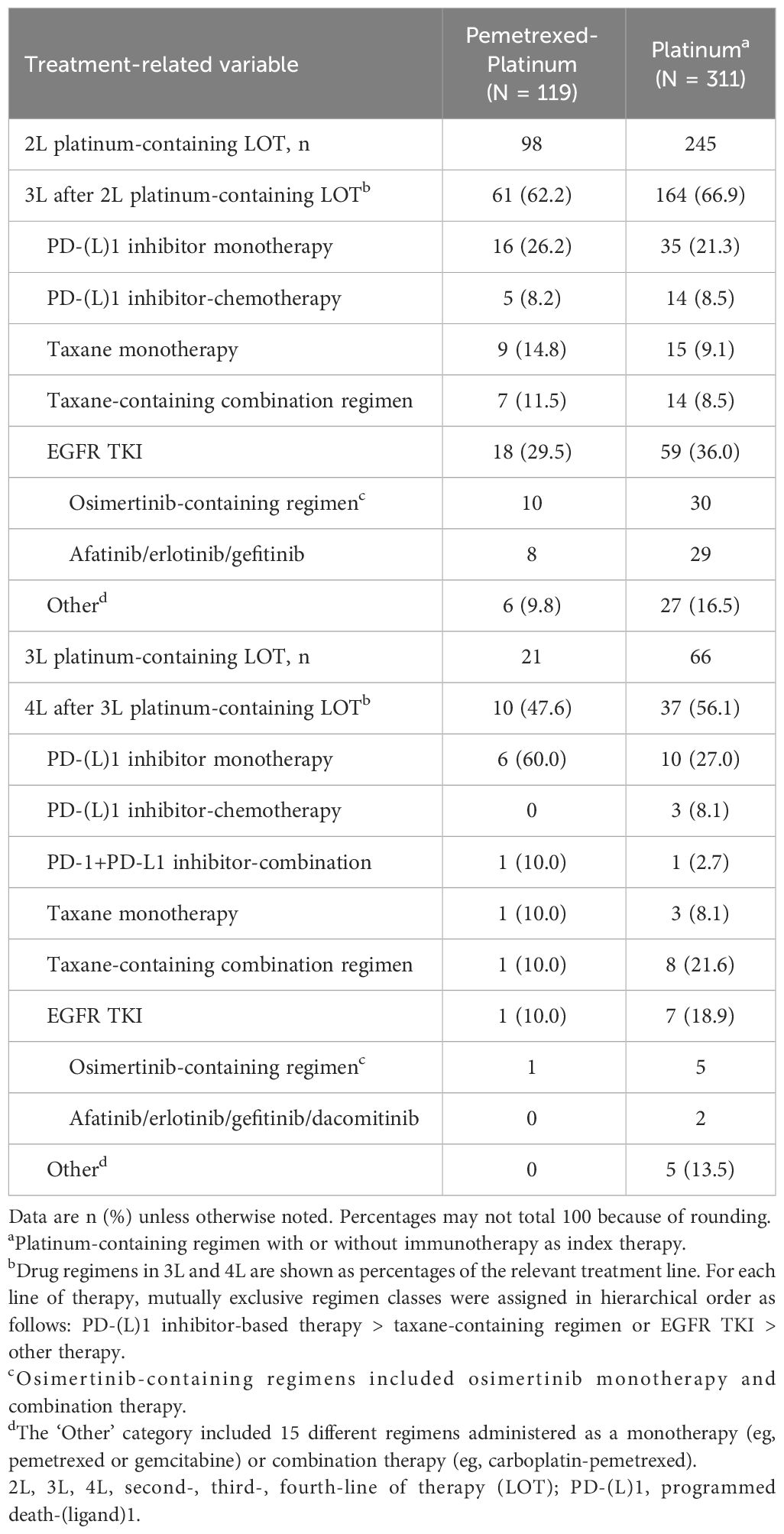
The median follow-up from the index date to data cutoff was 65 and 59 months in pemetrexed-platinum and platinum cohorts, respectively, while median patient follow-up from the index date to the earliest of death or data cutoff was 9 and 11 months, respectively (Table 2).
Median OS from initiation of pemetrexed-platinum was 10.3 months (95% CI, 8.1–13.9), and the survival rates at 12 and 24 months were 48% and 23%, respectively (Table 2, Figure 2). In the platinum cohort, median OS was 12.4 months (95% CI, 10.2–15.2), and the survival rates at 12 and 24 months were 51% and 28%, respectively.
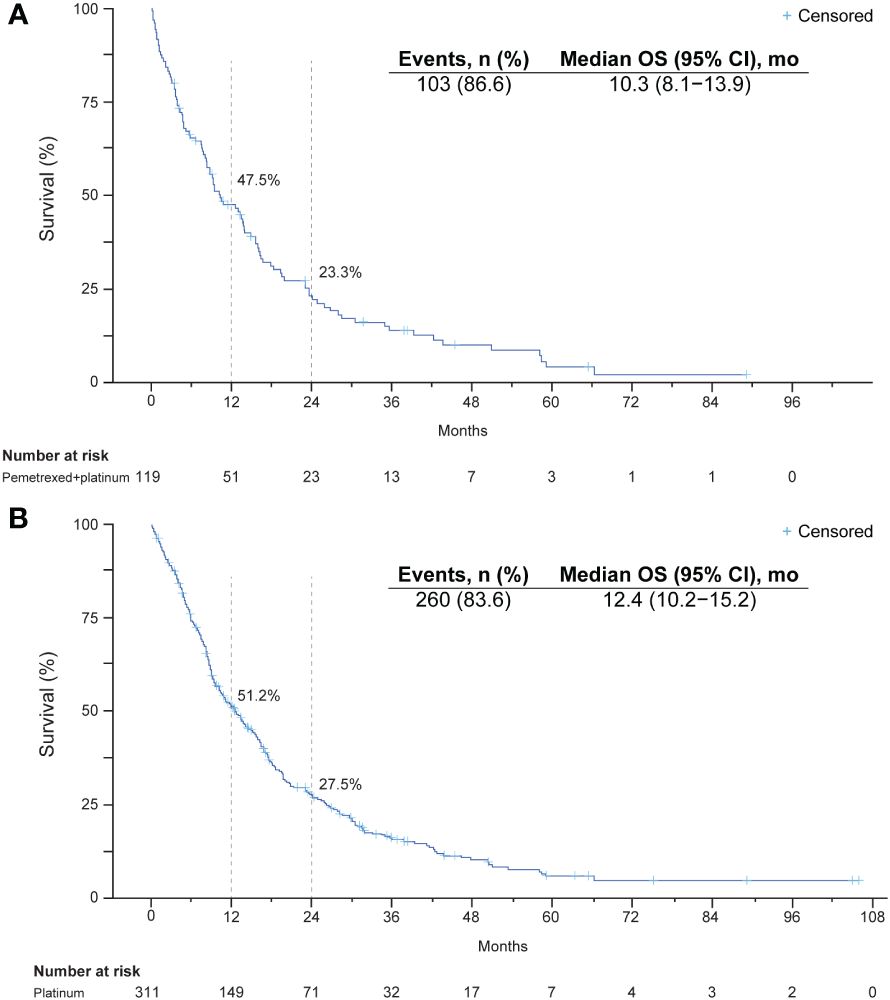
Figure 2 Kaplan-Meier plots of overall survival after initiation of a platinum-containing regimen: (A) platinum-pemetrexed combination regimen and (B) platinum-based regimen with or without immunotherapy.
Among the patients with ECOG PS of 0 or 1, median survival was 13.6 months in the pemetrexed-platinum cohort and 14.5 months in the platinum cohort (Table 2).
3.3 Systemic treatment regimens received after platinum-containing regimens
Seventy-one of 119 patients (60%) in the pemetrexed-platinum cohort and 201 of 311 patients (65%) in the platinum cohort received another systemic therapy after the platinum-containing regimen. These included PD-(L)1 inhibitor-, EGFR TKI-, and taxane-based regimens (Table 3).
4 Discussion
This retrospective study followed patients treated in the real-world setting of US oncology practices for advanced nonsquamous NSCLC with sensitizing EGFR mutations (ex19del/L858R) and who initiated platinum-containing chemotherapy from 2011 to mid-2020 after having received one or two lines of EGFR TKI therapy, with follow-up to mid-2022. Median OS after initiating pemetrexed-platinum was 10.3 months and after platinum, 12.4 months; 12-month survival rates were 48% and 51%, respectively. Outcomes were somewhat better for patients with good performance status, namely, median OS of 13.6 months and 14.5 months after pemetrexed-platinum and platinum initiation, respectively, with 12-month survival rates of 56% in both cohorts. Subsequent third- and fourth-line therapies were varied and included PD-(L)1 inhibitor-based, taxane-based, and EGFR TKI-based regimens.
Real-world outcomes were also examined in a small retrospective study of 135 patients with EGFR-mutated advanced NSCLC who received subsequent therapy in 2015 to 2021 after EGFR TKIs. Treated at two tertiary cancer centers in the Netherlands, these patients received a variety of chemotherapy regimens; and median OS was 15.3 months (95% CI, 11.6–18.9), slightly longer than in the present study, with no significant difference detected among regimens, the most common of which was pemetrexed-platinum (36).
In a recent US real-world study, Nieva et al. used the CancerLinQ database to examine treatment patterns and survival of patients with EGFR-mutated advanced NSCLC diagnosed in 2011–2018 who received first-line therapy with a first- or second-generation EGFR TKI (29). Median OS from the start of second-line treatment was longer for the 186 patients who received osimertinib in second-line (28.9 months) than for the 353 who received other therapies (13.0 months), the minority of whom received platinum-based chemotherapy (as in our study). Of note, in their study, 28% of patients died before initiating second-line treatment, and only 52% of patients continued to second-line. Similar to our study, NSCLC was diagnosed at an advanced stage for the majority of patients (29). Other recent US real-world studies of patients with EGFR-mutated NSCLC have described biomarker testing patterns and/or treatment patterns for first-line therapy and disease progression after first line (26–28, 37–41).
In recently published clinical trials of similar patient populations who experienced disease progression after EGFR TKI therapy (11–13), the median OS was somewhat longer in the pemetrexed-platinum arms (17.9, 18.7, and 19.5 months) relative to our findings for patients with ECOG PS of 0 or 1, albeit with the caveat that fewer than 50 patients were included in the treatment arms of two of the trials (12, 13). However, a better outcome in clinical trials (relative to our real-world findings) is not an unexpected finding, because of more frequent front-line osimertinib and the fact that trial enrollment criteria tend to select for patients without comorbidity or concomitant therapies, different from an unselected real-world patient population (24).
The patient population included in the present study was representative of a population with EGFR-mutated advanced nonsquamous NSCLC, namely, including more women (66%) than men, more Asian patients (15%) than in other US real-world studies not restricted to EGFR-mutated NSCLC, and more nonsmokers (57%) than smokers (3, 6, 42). We studied over 300 patients and treatment outcomes over a decade during which EGFR TKIs became the standard of care as first-line therapy for EGFR-mutated NSCLC. Designed with a minimum of 2 years of potential follow-up (until 30 June 2022), our study used a well-maintained database that is frequently used for oncology research (30–32).
We note several study limitations, however. Our findings may have limited generalizability to academic compared with community centers and to centers outside the Flatiron Health network (43, 44). A small percentage of Black patients (≤8%), fewer than the US population percentage of 14%, were included in the database, and ECOG PS data were missing for approximately one-third of patients. Testing for PD-L1 expression was not introduced until 2015; therefore, incomplete availability of PD-L1 expression status was not unexpected. Consistent with clinical guidelines, few patients received concomitant immunotherapy. However, the numbers (87/311) were insufficient for outcomes analysis in these patients. Finally, more recent real-world data would likely reveal different treatment patterns as osimertinib is now standard of care in front line, and further observational studies of patients treated in real-world settings are needed.
Research continues to identify the optimal systemic regimens for patients with TKI-resistant EGFR-mutated NSCLC. While the role of immunotherapy remains uncertain (14), several ongoing trials are investigating novel therapies, such as antibody-drug conjugates, to improve outcomes for these patients (45).
5 Conclusions
Survival outcomes in this real-world study of patients with advanced nonsquamous NSCLC who experienced disease progression after EGFR TKI therapy appeared shorter than, but within the range of, historical clinical trials. The wide variability in platinum-containing regimens and in regimens administered as the next line of therapy is indicative of the lack of treatment regimens with proven efficacy for this population. The suboptimal survival outcomes recorded in this study demonstrate the unmet need to identify more effective subsequent treatment regimens for patients with EGFR-mutated advanced nonsquamous NSCLC after EGFR TKI resistance develops.
Data availability statement
The data that support the findings of this study have been originated by Flatiron Health, Inc. Requests for data sharing by license or by permission for the specific purpose of replicating results in this article can be submitted to publicationsdataaccess@flatiron.com.
Ethics statement
Institutional Review Board approval of the study protocol was obtained from WCG Institutional Review Board before conducting the study and included a waiver of informed consent for working with deidentified data. The deidentified data were subject to obligations to prevent reidentification during the analyses to protect patient confidentiality.
Author contributions
BH: Writing – review & editing, Visualization, Validation. PR: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Conceptualization. JM: Writing – review & editing, Validation, Investigation, Formal analysis, Data curation. XH: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Conceptualization. DC: Writing – review & editing, Visualization, Validation, Methodology, Conceptualization. MS: Writing – review & editing, Visualization, Validation. BZ: Writing – review & editing, Visualization, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Acknowledgments
We gratefully acknowledge Weilin Meng for analytic support and Mary Anne Rutkowski for programming support (both of Merck & Co., Inc., Rahway, NJ, USA). Medical writing and editorial assistance were provided by Elizabeth V. Hillyer, DVM (freelance). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Conflict of interest
BH reports grants from Boehringer Ingelheim, Astra Zeneca, Merck, BMS, Advaxis, Amgen, AbbVie, Daiichi, Pfizer, GSK, Beigene, and Janssen; consulting fees from AstraZeneca, Boehringer Ingelheim, Janssen, Takeda, Merck, BMS, Genentech, Pfizer, Eli-Lilly, Arcus, and Merus; and participation in a Data Safety Monitoring Board or Advisory Board for TPT, BMS, Apollomics, Nuvalent, and Merck. PR, JM, XH, DC, MS, and BZ report full-time employment with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stock ownership of Merck & Co., Inc., Rahway, NJ, USA.
The authors declare that this study received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Employees of the funder participated in the study design; in the collection, analysis, and interpretation of data; in the writing of this article; and in the decision to submit the article for publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1285280/full#supplementary-material
References
1. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. (2020) 383:640–9. doi: 10.1056/NEJMoa1916623
2. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. (2014) 9:154–62. doi: 10.1097/JTO.0000000000000033
3. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. (2015) 5:2892–911.
4. Graham RP, Treece AL, Lindeman NI, Vasalos P, Shan M, Jennings LJ, et al. Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med. (2018) 142:163–7. doi: 10.5858/arpa.2016-0579-CP
5. D'Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. (2011) 29:2066–70. doi: 10.1200/JCO.2010.32.6181
6. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. (2022) 40:611–25. doi: 10.1200/JCO.21.01626
7. Keedy VL, Temin S, Somerfield MR, Beasley MB, Johnson DH, McShane LM, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. (2011) 29:2121–7. doi: 10.1200/JCO.2010.31.8923
8. Piotrowska Z, Sequist LV. Treatment of EGFR-mutant lung cancers after progression in patients receiving first-line EGFR tyrosine kinase inhibitors : a review. JAMA Oncol. (2016) 2:948–54. doi: 10.1001/jamaoncol.2016.0333
9. Owen DH, Singh N, Ismaila N, Masters G, Riely GJ, Robinson AG, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO living guideline, version 2023.2. J Clin Oncol. (2023) 41:e63–72. doi: 10.1200/JCO.23.01055
10. Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol. (2022) 19:499–514. doi: 10.1038/s41571-022-00639-9
11. Mok TSK, Kim SW, Wu YL, Nakagawa K, Yang JJ, Ahn MJ, et al. Gefitinib plus chemotherapy versus chemotherapy in epidermal growth factor receptor mutation-positive non-small-cell lung cancer resistant to first-line gefitinib (IMPRESS): overall survival and biomarker analyses. J Clin Oncol. (2017) 35:4027–34. doi: 10.1200/JCO.2017.73.9250
12. Wu YL, Cheng Y, Zhou J, Lu S, Zhang Y, Zhao J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med. (2020) 8:1132–43. doi: 10.1016/S2213-2600(20)30154-5
13. Yoo KH, Lee SJ, Cho J, Lee KH, Park KU, Kim KH, et al. A randomized, open-label, phase II study comparing pemetrexed plus cisplatin followed by maintenance pemetrexed versus pemetrexed alone in patients with epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer after failure of first-line EGFR tyrosine kinase inhibitor: KCSG-LU12-13. Cancer Res Treat. (2019) 51:718–26. doi: 10.4143/crt.2018.324
14. Muthusamy B, Pennell N. Chemoimmunotherapy for EGFR-mutant NSCLC: still no clear answer. J Thorac Oncol. (2022) 17:179–81. doi: 10.1016/j.jtho.2021.11.012
15. Mok T, Nakagawa K, Park K, Ohe Y, Girard N, Kim HR, et al. Nivolumab plus chemotherapy in epidermal growth factor receptor-mutated metastatic non-small-cell lung cancer after disease progression on epidermal growth factor receptor tyrosine kinase inhibitors: final results of CheckMate 722. J Clin Oncol. (2024) JCO2301017. doi: 10.1200/JCO.23.01017
16. Lee ATM, Nagasaka M. CheckMate-722: The rise and fall of nivolumab with chemotherapy in TKI-refractory EGFR-mutant NSCLC. Lung Cancer Targets Ther. (2023) 14:41–6. doi: 10.2147/LCTT.S408886
17. Riely GJ, Hui R, Carbone DP, Park K, Carrigan M, Xu X, et al. P1.01-81 Phase 3 study of pemetrexed-platinum with or without pembrolizumab for TKI-resistant/EGFR-mutated advanced NSCLC: KEYNOTE-789 (abstract). J Thorac Oncol. (2018) 13:S494. doi: 10.1016/j.jtho.2018.08.637
18. Yang JC-H, Lee DH, Lee J-S, Fan Y, de Marinis F, Okamoto I, et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR-mutant, metastatic nonsquamous NSCLC: Phase 3 KEYNOTE-789 study (abstract). J Clin Oncol. (2023) 41. doi: 10.1200/JCO.2023.41.17_suppl.LBA9000. abstr LBA9000.
19. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
20. Lu S, Wu L, Jian H, Chen Y, Wang Q, Fang J, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:1167–79. doi: 10.1016/S1470-2045(22)00382-5
21. Lu S, Wu L, Jian H, Cheng Y, Wang Q, Fang J, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. (2023) 11:624–36. doi: 10.1016/S2213-2600(23)00135-2
22. Singh N, Jaiyesimi IA, Ismaila N, Leighl NB, Mamdani H, Phillips T, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO Living Guideline, version 2023.1. J Clin Oncol. (2023) 41:e51–62. doi: 10.1200/JCO.23.00282
23. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.3.2023. © National Comprehensive Cancer Network, Inc (2023). All rights reserved. Accessed June 6, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
24. Nazha B, Yang JC, Owonikoko TK. Benefits and limitations of real-world evidence: lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol. (2021) 17:965–77. doi: 10.2217/fon-2020-0951
25. Gaitonde P, Chirikov V, Kelkar S, Liljas B. Considerations for the utility of real-world evidence beyond trial data in advanced NSCLC: The case of frontline tyrosine kinase inhibitors. Cancer Manag Res. (2022) 14:3421–35. doi: 10.2147/CMAR.S380857
26. Chiang AC, Fernandes AW, Pavilack M, Wu JW, Laliberte F, Duh MS, et al. EGFR mutation testing and treatment decisions in patients progressing on first- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer. (2020) 20:356. doi: 10.1186/s12885-020-06826-0
27. Nadler E, Vasudevan A, Wang Y, Ogale S. Real-world patterns of biomarker testing and targeted therapy in de novo metastatic non-small cell lung cancer patients in the US oncology network. Cancer Treat Res Commun. (2022) 31:100522. doi: 10.1016/j.ctarc.2022.100522
28. Shenolikar R, Liu S, Shah A, Tse J, Cao Y, Near A. Real-world treatment patterns of metastatic non-small cell lung cancer patients receiving epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Med. (2023) 12:159–69. doi: 10.1002/cam4.4918
29. Nieva J, Reckamp KL, Potter D, Taylor A, Sun P. Retrospective analysis of real-world management of EGFR-mutated advanced NSCLC, after first-line EGFR-TKI treatment: US treatment patterns, attrition, and survival data. Drugs Real World Outcomes. (2022) 9:333–45. doi: 10.1007/s40801-022-00302-w
30. Khozin S, Miksad RA, Adami J, Boyd M, Brown NR, Gossai A, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer. (2019) 125:4019–32. doi: 10.1002/cncr.32383
31. Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.16.20037143v3
32. Birnbaum B, Nussbaum N, Seidl-Rathkopf K, Agrawal M, Estevez M, Estola E, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv [Preprint]. (2020). doi: 10.48550/arXiv.2001.09765
33. Curtis MD, Griffith SD, Tucker M, Taylor MD, Capra WB, Carrigan G, et al. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv Res. (2018) 53:4460–76. doi: 10.1111/1475-6773.12872
34. Carrigan G, Whipple S, Taylor MD, Torres AZ, Gossai A, Arnieri B, et al. An evaluation of the impact of missing deaths on overall survival analyses of advanced non-small cell lung cancer patients conducted in an electronic health records database. Pharmacoepidemiol Drug Saf. (2019) 28:572–81. doi: 10.1002/pds.4758
35. Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res. (2021) 56:1281–7. doi: 10.1111/1475-6773.13669
36. Steendam CMJ, Ernst SM, Badrising SK, Paats MS, Aerts J, de Langen AJ, et al. Chemotherapy for patients with EGFR-mutated NSCLC after progression on EGFR-TKI's: Exploration of efficacy of unselected treatment in a multicenter cohort study. Lung Cancer. (2023) 181:107248. doi: 10.1016/j.lungcan.2023.107248
37. Aguilar KM, Winfree KB, Muehlenbein CE, Zhu YE, Wilson T, Wetmore S, et al. Treatment patterns by EGFR mutation status in non-small cell lung cancer patients in the USA: a retrospective database analysis. Adv Ther. (2018) 35:1905–19. doi: 10.1007/s12325-018-0811-0
38. Soo RA, Seto T, Gray JE, Thiel E, Taylor A, Sawyer W, et al. Treatment patterns in patients with locally advanced or metastatic non-small-cell lung cancer treated with epidermal growth factor receptor-tyrosine kinase inhibitors: analysis of US insurance claims databases. Drugs Real World Outcomes. (2022) 9:31–41. doi: 10.1007/s40801-021-00272-5
39. Hess LM, Krein PM, Haldane D, Han Y, Sireci AN. Biomarker testing for patients with advanced/metastatic nonsquamous NSCLC in the United States of America, 2015 to 2021. JTO Clin Res Rep. (2022) 3:100336. doi: 10.1016/j.jtocrr.2022.100336
40. Winfree KB, Molife C, Peterson PM, Chen Y, Visseren-Grul CM, Leusch MS, et al. Real-world characteristics and outcomes of advanced non-small-cell lung cancer patients with EGFR exon 19 deletions or exon 21 mutations. Future Oncol. (2021) 17:2867–81. doi: 10.2217/fon-2021-0218
41. Winfree KB, Sheffield KM, Cui ZL, Sugihara T, Feliciano J. Study of patient characteristics, treatment patterns, EGFR testing patterns and outcomes in real-world patients with EGFRm(+) non-small cell lung cancer. Curr Med Res Opin. (2022) 38:91–9. doi: 10.1080/03007995.2021.1983530
42. Khozin S, Abernethy AP, Nussbaum NC, Zhi J, Curtis MD, Tucker M, et al. Characteristics of real-world metastatic non-small cell lung cancer patients treated with nivolumab and pembrolizumab during the year following approval. Oncologist. (2018) 23:328–36. doi: 10.1634/theoncologist.2017-0353
43. Ramalingam S, Dinan MA, Crawford J. Survival comparison in patients with stage IV lung cancer in academic versus community centers in the United States. J Thorac Oncol. (2018) 13:1842–50. doi: 10.1016/j.jtho.2018.09.007
44. Ramalingam S, Dinan MA, Crawford J. Treatment at integrated centers might bridge the academic-community survival gap in patients with metastatic non-small cell carcinoma of the lung. Clin Lung Cancer. (2021) 22:e646–e53. doi: 10.1016/j.cllc.2020.12.013
Keywords: advanced non-small cell lung cancer, sensitizing EGFR mutation, overall survival, platinum-containing chemotherapy, subsequent therapy, tyrosine kinase inhibitor
Citation: Halmos B, Rai P, Min J, Hu X, Chirovsky D, Shamoun M and Zhao B (2024) Real-world outcomes on platinum-containing chemotherapy for EGFR-mutated advanced nonsquamous NSCLC with prior exposure to EGFR tyrosine kinase inhibitors. Front. Oncol. 14:1285280. doi: 10.3389/fonc.2024.1285280
Received: 29 August 2023; Accepted: 18 March 2024;
Published: 18 April 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Lara Kujtan, University of Missouri–Kansas City, United StatesHidehito Horinouchi, National Cancer Center Hospital, Japan
Copyright © 2024 Halmos, Rai, Min, Hu, Chirovsky, Shamoun and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pragya Rai, Pragya.rai@merck.com
 Balazs Halmos
Balazs Halmos Pragya Rai
Pragya Rai Jae Min2
Jae Min2 Xiaohan Hu
Xiaohan Hu Diana Chirovsky
Diana Chirovsky Bin Zhao
Bin Zhao