Abstract
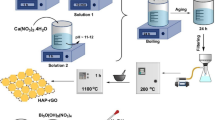
The present work focuses on the synthesis of Prosopis juliflora bark activated carbon supported on TiO2 (PJBAC/TiO2) composite through the sol-gel method for the decolourization of Direct Brown 2 (DB2). The prepared composite was characterized by Fourier-transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive X-ray (EDX) analysis. The surface area and pore diameter were explored by the Brunauer−Emmett−Teller method (N2 adsorption/desorption). Furthermore, high-performance liquid chromatography (HPLC) and total organic carbon (TOC) analysis of the treated solution revealed a complete degradation of the dye molecule. The degradation efficiency of the prepared composite was analysed via batch equilibration studies. Maximum removal of DB2 (98%) was achieved at an initial concentration of 100 mg/L, contact time of 210 min, composite dose of 100 mg, and at pH 3. The well-known Freundlich and Langmuir isotherm equations were applied for the evaluation of equilibrium adsorption data. Lagergren and Ho−McKay kinetic models were employed to determine the adsorption rate constant. Additionally, the antimicrobial activity of PJBAC/TiO2 was tested against Staphylococcus aureus, Escherichia coli, and Candida species. These results indicate that doping of TiO2 on PJBAC inhibits the recombination of electron−hole pairs to improve photocatalytic performance in the visible region.










Similar content being viewed by others
REFERENCES
Gupta, V.K., Kumar, R., Nayak, A., Saleh, T.A., and Barakat, M.A., Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review, Adv. Colloid Interface Sci., 2013, vols. 193–194, pp. 24–34. https://doi.org/10.1016/j.cis.2013.03.003
El-Berry, M.F., Sadeek, S.A., Abdalla, A.M., and Nassar, M.Y., Microwave-assisted fabrication of copper nanoparticles utilizing different counter ions: An efficient photocatalyst for photocatalytic degradation of safranin dye from aqueous media, Mater. Res. Bull., 2021, vol. 133, p. 111048. https://doi.org/10.1016/j.materresbull.2020.111048
Wu, Q., Li, W.-T., Yu, W.-H., Li, Y., and Li, A.-M., Removal of fluorescent dissolved organic matter in biologically treated textile wastewater by the ozonation-biological aerated filter, J. Taiwan Inst. Chem. Eng., 2016, vol. 59, pp. 359–364. https://doi.org/10.1016/j.jtice.2015.08.015
Mishra, A., Mehta, A., and Basu, S., Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review, J. Environ. Chem. Eng., 2018, vol. 6, no. 5, pp. 6088–6107. https://doi.org/10.1016/j.jece.2018.09.029
Slimen, H., Houas, A., and Nogier, J.P., Elaboration of stable anatase TiO2 through activated carbon addition with high photocatalytic activity under visible light, J. Photochem. Photobiol., A, 2011, vol. 221, no. 1, pp. 13–21. https://doi.org/10.1016/j.jphotochem.2011.04.013
Fagan, R., McCormack, D.E., Dionysiou, D.D., and Pillai, S.C., A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern, Mater. Sci. Semicond. Process., 2016, vol. 42, pp. 2–14. https://doi.org/10.1016/j.mssp.2015.07.052
Kumar, M. and Tamilarasan, R., Modeling of experimental data for the adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Prosopis juliflora, Polish J. Che. Tech., 2013, vol. 15, no. 2, pp. 9–39. https://doi.org/10.2478/pjct-2013-0021
Chapin, K.C. and Lauderdale, T., Reagents, stains, and media: Bacteriology, in Manual of Clinical Microbiology, Murray, P.R., Baron, E.J., Jorgensen, J.H., Pfaller, M.A., and Yolken, R.H., Eds., Washington, DC: ASM, 2003, 8th ed., p. 358. https://www.academia.edu/35816694/Clinical_Microbiology_Procedures_Handbook.
Yahiro, H., Miyamoto, T., Watanabe, N., and Yamaura, H., Photocatalytic partial oxidation of methylstyrene over TiO2 supported on zeolites, Catal. Today, 2007, vol. 120, pp. 158–162. https://doi.org/10.1016/j.cattod.2006.07.039
Liu, L., Hu, T., Dai, K., Zhang, J., and Liang, C., A novel step-scheme BiVO4/Ag3VO4 photocatalyst for enhanced photocatalytic degradation activity under visible light irradiation, Chin. J. Catal., 2021, vol. 42, pp. 46–55. https://doi.org/10.1016/S1872-2067(20)63560-4
Tripathi, R.M., Hameed, P., Rao, R.P., Shrivastava, N., Mittal, J., and Mohapatra, S., Biosynthesis of highly stable fluorescent selenium nanoparticles and the evaluation of their photocatalytic degradation of dye, J. Bionanosci., 2020, vol. 10, pp. 389–396. https://doi.org/10.1007/s12668-020-00718-0
Khaled, A., Nemr, A.E., El-Sikaily, A., and Abdelwahab, O., Removal of Direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: Adsorption isotherm and kinetic studies, J. Hazard. Mater., 2009, vol. 165, pp. 100–110. https://doi.org/10.1016/j.jhazmat.2008.09.122
Vaez, Z. and Javanbakht, V., Synthesis, characterization and photocatalytic activity of ZSM-5/ZnO nanocomposite modified by Ag nanoparticles for Methyl Orange degradation. J. Photochem. Photobiol. Chem., 2019, vol. 388, p. 112064. https://doi.org/10.1016/j.jphotochem.2019.112064
Mahadwad, O.K., Parikh, P.A., Jasra R.V., and Patil, C., Photocatalytic degradation of Reactive Black-5 dye using TiO2 impregnated ZSM-5, Bull. Mater. Sci., 2011, vol. 34, no. 3, pp. 551–556. https://doi.org/10.1007/s12034-011-0124-2
Rezaee, A., Ghaneian, M.T., Taghavinia, N., Aminian M.K., and Hashemian, S.J., TiO2 nanofibre assisted photocatalytic degradation of Reactive Blue 19 dye from aqueous solution, Environ. Technol., 2009, vol. 30, pp. 233–239. https://doi.org/10.1080/09593330802630777
Sari, A. and Tuzen, M., Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass, J. Hazard. Mater., 2008, vol. 152, no. 1, pp. 302–308. https://doi.org/10.1016/j.jhazmat.2007.06.097
Heibati, B., Rodriguez-Couto, S., Al-Ghouti, M.A., Asif, M., Tyagi, I., Agarwal, S., and Gupta, V.K., Kinetics and thermodynamics of enhanced adsorption of the dye AR 18 using activated carbons prepared from walnut and poplar woods, J. Mol. Liq., 2015, vol. 208, pp. 99–105. https://doi.org/10.1016/j.molliq.2015.03.057
Arfaoui, S., Srasra, N.F., and Srasra, E., Modelling of the adsorption of the chromium ion by modified clays, Desalination, 2008, vol. 222, pp. 474–481. https://doi.org/10.1016/j.desal.2007.03.014
Yardim, M.F., Budinova, T., Ekinci, E., Petrov, N., Razvigorova, M., and Minkoba, V., Removal of mercury(II) from aqueous solution by activated carbon obtained from furfural, Chemosphere, 2003, vol. 52, no. 5, pp. 835–841. https://doi.org/10.1016/s0045-6535(03)00267-4
Ho, Y.S. and McKay, G., Kinetic models for the sorption of dye from aqueous solution by wood, Process Saf. Environ. Prot., 1998, vol. 76, pp. 183–191. https://doi.org/10.1205/095758298529326
Li, R., Che, T., and Pan, X., Metal-organic-framework-based materials for antimicrobial applications, ACS Nano, 2021, vol. 15, no. 3, pp. 3808–3848. https://doi.org/10.1021/acsnano.0c09617
Thirumagal, N. and Jeyakumari, A.P., Photocatalytic and antibacterial activities of AgNPs from Mesua ferrea seed, SN Appl. Sci., 2020, vol. 2, p. 2064. https://doi.org/10.1007/s42452-020-03650-w
Tahmasebizad, N., Hamedani, M.T., Ghazani, M.S., and Pazhuhanfar, Y., Photocatalytic activity and antibacterial behavior of TiO2 coatings co-doped with copper and nitrogen via sol–gel method, J. Sol-gel. Sci. Technol., 2020, vol. 93, no. 3, pp. 570–578. https://doi.org/10.1007/s10971-019-05085-1
Prabakaran, M., Kim, S.-H., Sasireka, A., Chandrasekaran, M., and Chung, I.-M., Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera, Food Biosci., 2018, vol. 26, pp. 23–29. https://doi.org/10.1016/j.fbio.2018.09.003
Radha, V.P. and Prabakaran, M., Novel thiadiazole-derived Schiff base ligand and its transition metal complexes: Thermal behaviour, theoretical study, chemo-sensor, antimicrobial, antidiabetic and anticancer activity, A-ppl. Organomet. Chem., 2022, vol. 36, no. 11, p. e6872. https://doi.org/10.1002/aoc.6872
Arunadevi, N., Kanchana, P., Hemapriya, V., Mehala, M., Swathika, M., Chung, I.-M., and Prabakaran, M., A two-step strategy to synthesis new aminoguanidinium complexes: Cytotoxic effect and perspectives, Inorg. Nano-Met. Chem., 2022. https://doi.org/10.1080/24701556.2022.2081193
ACKNOWLEDGMENTS
The authors are thankful to the Department of Chemistry, Dr.N.G.P Institute of Technology, Coimbatore, Tamil Nadu, India for their kind support in carrying out these studies.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kallapalayam Subramaniam Thangamani, Suba, V., Radha, V.P. et al. Investigation on Nanoarchitectonics of PJBAC/TiO2 for Photocatalytic and Antimicrobial Performance. J. Water Chem. Technol. 46, 132–148 (2024). https://doi.org/10.3103/S1063455X24020127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X24020127