Abstract
Backgroud
Granulocyte colony-stimulating factor (G-CSF) is widely used for the primary prophylaxis of febrile neutropenia (FN). Two types of G-CSF are available in Japan, namely G-CSF chemically bound to polyethylene glycol (PEG G-CSF), which provides long-lasting effects with a single dose, and non-polyethylene glycol-bound G-CSF (non-PEG G-CSF), which must be sequentially administrated for several days.
Methods
This current study investigated the utility of these treatments for the primary prophylaxis of FN through a systematic review of the literature. A detailed literature search for related studies was performed using PubMed, Ichushi-Web, and the Cochrane Library. Data were independently extracted and assessed by two reviewers. A qualitative analysis or meta-analysis was conducted to evaluate six outcomes.
Results
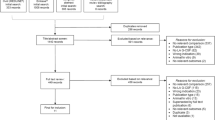
Through the first and second screenings, 23 and 18 articles were extracted for qualitative synthesis and meta-analysis, respectively. The incidence of FN was significantly lower in the PEG G-CSF group than in the non-PEG G-CSF group with a strong quality/certainty of evidence. The differences in other outcomes, such as overall survival, infection-related mortality, the duration of neutropenia (less than 500/μL), quality of life, and pain, were not apparent.
Conclusions
A single dose of PEG G-CSF is strongly recommended over multiple-dose non-PEG G-CSF therapy for the primary prophylaxis of FN.



Similar content being viewed by others
Data availability
Data associated with this systematic review can be accessed from the corresponding author upon reasonable request.
References
Crawford J, Caserta C, Roila F et al (2010) Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. https://doi.org/10.1093/annonc/mdq195
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 47(1):8–32. https://doi.org/10.1016/j.ejca.2010.10.013
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33(28):3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
NCCN Clinical Practice Guidelines in Oncology. Hematopoietic growth factors Version 2. 2023
Yang BB, Kido A (2011) Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet 50(5):295–306. https://doi.org/10.2165/11586040-000000000-00000
Morizane T, Yoshida M, Kojimahara N, et al (2014) Minds handbook for clinical practice guideline development 2014. Japan Council for Quality Health Care, Tokyo. https://minds.jcqhc.or.jp/s/developer_manual(in Japanese)
Kojimahara N, Nakayama T, Morizane T, et al (2017) Minds manual for guideline development 2017. Japan Council for Quality Health Care, Tokyo
Cesaro S, Nesi F, Tridello G et al (2013) A randomized, non-inferiority study comparing efficacy and safety of a single dose of pegfilgrastim versus daily filgrastim in pediatric patients after autologous peripheral blood stem cell transplant. PLoS ONE 8(1):e53252. https://doi.org/10.1371/journal.pone.0053252
Holmes FA, Jones SE, O’Shaughnessy J et al (2002) Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol 13(6):903–909. https://doi.org/10.1093/annonc/mdf130
Holmes FA, O’Shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20(3):727–731. https://doi.org/10.1200/JCO.2002.20.3.727
Green MD, Koelbl H, Baselga J et al (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14(1):29–35. https://doi.org/10.1093/annonc/mdg019
Grigg A, Solal-Celigny P, Hoskin P et al (2003) Open-label, randomized study of pegfilgrastim vs daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin’s lymphoma. Leuk Lymphoma 44(9):1503–1508. https://doi.org/10.1080/1042819031000103953
Siena S, Piccart MJ, Holmes FA et al (2003) A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily Filgrastim in patients with stage II-IV breast cancer. Oncol Rep 10(3):715–724
Vose JM, Crump M, Lazarus H et al (2003) Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol 21(3):514–519. https://doi.org/10.1200/JCO.2003.03.040
Sierra J, Szer J, Kassis J et al (2008) A single dose of pegfilgrastim compared with daily filgrastim for supporting neutrophil recovery in patients treated for low-to-intermediate risk acute myeloid leukemia: results from a randomized, double-blind, phase 2 trial. BMC Cancer 10(8):195. https://doi.org/10.1186/1471-2407-8-195
Fox E, Widemann BC, Hawkins DS et al (2009) Randomized trial and pharmacokinetic study of pegfilgrastim versus filgrastim after dose-intensive chemotherapy in young adults and children with sarcomas. Clin Cancer Res 15(23):7361–7367. https://doi.org/10.1158/1078-0432.CCR-09-0761
Rifkin R, Spitzer G, Orloff G et al (2010) Pegfilgrastim appears equivalent to daily dosing of filgrastim to treat neutropenia after autologous peripheral blood stem cell transplantation in patients with non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 10(3):186–191. https://doi.org/10.3816/CLML.2010.n.029
Spunt SL, Irving H, Frost J et al (2010) Phase II, randomized, open-label study of pegfilgrastim-supported VDC/IE chemotherapy in pediatric sarcoma patients. J Clin Oncol 28(8):1329–1336. https://doi.org/10.1200/JCO.2009.24.8872
Zhang W, Jiang Z, Wang L et al (2015) An open-label, randomized, multicenter dose-finding study of once-per-cycle pegfilgrastim versus daily filgrastim in Chinese breast cancer patients receiving TAC chemotherapy. Med Oncol 32(5):147. https://doi.org/10.1007/s12032-015-0537-7
Kubo K, Miyazaki Y, Murayama T et al (2016) A randomized, double-blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br J Haematol 174(4):563–570. https://doi.org/10.1111/bjh.14088
Martino M, Praticò G, Messina G et al (2006) Pegfilgrastim compared with filgrastim after high-dose melphalan and autologous hematopoietic peripheral blood stem cell transplantation in multiple myeloma patients. Eur J Haematol 77(5):410–415. https://doi.org/10.1111/j.1600-0609.2006.00736.x
Martino M, Gori M, Tripepi G et al (2020) A comparative effectiveness study of lipegfilgrastim in multiple myeloma patients after high dose melphalan and autologous stem cell transplant. Ann Hematol 99(2):331–341. https://doi.org/10.1007/s00277-019-03901-w
Tadmor T, Levy I, Herishanu Y et al (2019) Primary peg-filgrastim prophylaxis versus filgrastim given “on demand” for neutropenia during therapy with cladribine for hairy cell leukemia. Leuk Res 82:24–28. https://doi.org/10.1016/j.leukres.2019.05.006
Kourlaba G, Dimopoulos MA, Pectasides D et al (2015) Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer 23(7):2045–2051. https://doi.org/10.1007/s00520-014-2555-y
Almenar D, Mayans J, Juan O et al (2009) Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain–results of the LEARN Study. Eur J Cancer Care (Engl) 18(3):280–286. https://doi.org/10.1111/j.1365-2354.2008.00959.x
Staber PB, Holub R, Linkesch W et al (2005) Fixed-dose single administration of Pegfilgrastim vs daily Filgrastim in patients with haematological malignancies undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 35(9):889–893. https://doi.org/10.1038/sj.bmt.1704927
Gerds A, Fox-Geiman M, Dawravoo K et al (2010) Randomized phase III trial of pegfilgrastim versus filgrastim after autologus peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 16(5):678–685. https://doi.org/10.1016/j.bbmt.2009.12.531
Henk HJ, Becker L, Tan H et al (2013) Comparative effectiveness of pegfilgrastim, filgrastim, and sargramostim prophylaxis for neutropenia-related hospitalization: two US retrospective claims analyses. J Med Econ 16(1):160–168. https://doi.org/10.3111/13696998.2012.734885
Kubista E, Glaspy J, Holmes FA et al (2003) Bone pain associated with once-per-cycle pegfilgrastim is similar to daily filgrastim in patients with breast cancer. Clin Breast Cancer 3(6):391–398. https://doi.org/10.3816/cbc.2003.n.003
Acknowledgements
We are grateful to Mr. Naohiko Yamaguchi for his contribution to the initial literature search. We would also like to thank Ms. Natsuki Fukuda for their valuable comments and suggestions. We additionally thank Joe Barber Jr., PhD, from Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Funding
The present study did not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the present study. The first draft of the manuscript was written by T.Y., and all authors commented on the previous version of the manuscript. All authors read and gave their final approval.
Corresponding author
Ethics declarations
Conflict of interest
TE.Y. received honoraria from Kyowa-Kirin, Pfizer, Chugai, Eli Lilly, MSD, Astra Zeneca and Eisai. Y.O. received honoraria from Daiichi-Sankyo, Pfizer, Chugai, Lilly and Kyowa Kirin. K.T. received honoraria from Ono, Chugai, Taiho and Novartis. E.I. received honoraria from Eli Lilly, and research funding from MSD, Ono, Janssen Pharma and Takeda. Y.M. received honoraria from Ono, MSD, Takeda, Eisai and Bristol Myers Squibb, and research funding from MSD and Ono. S.Y. received research funding from Otsuka. D.M. received honoraria from Janssen, Nippon Shinyaku, Eisai, Mundipharma, Kyowa Kirin, Chugai, Zenyaku, MSD, SymBio, Sanofi, AbbVie, Takeda, Astra Zeneca and Bristol Myers Squibb, and research funding from Biopharma, Novartis, Kyowa Kirin, Ono, Chugai, Janssen, Takeda, Otsuka, Sanofi, Astellas, Bristol Myers Squibb, AbbVie, Eisai, MSD, Taiho and Astra Zeneca. T.M. received honoraria from Astra Zeneca, Chugai and Myriadgenetics. E.B. received honoraria from Chugai, and Daiichi-Sankyo, and research funding from Taiho and Chugai. T.KU. received honoraria from Chugai. T.KI. received honoraria from Sanofi. S.N. received honoraria from Kyowa Kirin. A.S. received honoraria and scholarship donation from Chugai and Taiho. T.T. received honoraria from Daiichi-Sankyo, Chugai and Eli Lilly. Other authors declare that there are no conflicts of interest associated with this manuscript.
Ethical approval
Not applicable.
Informed consent
Formal consent was not required for this type of study.
Consent to participate
Not applicable.
Consent for publication
All authors consented to the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yoshinami, T., Nozawa, K., Yokoe, T. et al. Comparison between a single dose of PEG G-CSF and multiple doses of non-PEG G-CSF: a systematic review and meta-analysis from Clinical Practice Guidelines for the use of G-CSF 2022. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02504-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02504-4