Abstract
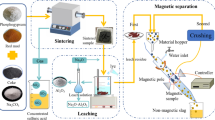
Red mud and spent cathode carbon block (SCCB) are environmentally hazardous industrial wastes generated by the alumina and aluminum electrolysis industries, respectively. In this paper, SCCB was applied to iron recovery from high-iron red mud by vortex smelting reduction with the concept of ‘waste treatment by waste.’ The experimental results showed that the SCCB can effectively replace the reducing agent such as coking coal to realize the recovery of iron from high-iron red mud. Pig iron was obtained under the optimal reduction conditions of an experimental temperature of 1350 °C, alkalinity of 1.0, carbon ratio of 1.0, and time of 20 min. More than 91% of the iron was recovered. In addition, during the reduction process, prolonging the holding time, increasing the temperature, and increasing the alkalinity were favorable to removing Na. The Na in the reduced slag was mainly in the form of NaAlSiO4. The increase in holding time favored the generation of the NaAlSiO4 phase, while the increase in alkalinity had the opposite effect, and changes in temperature and carbon ratio had little effect on the Na-containing phase. Finally, the fluoride ion concentration in the leach solution of reduction slag was 24.7 mg/L (lower than 100 mg/L), which proved that the reduction tailings could effectively realize fluoride fixation.
Graphical Abstract











Similar content being viewed by others
References
Khairul MA, Zanganeh J, Moghtaderi B (2019) The composition, recycling and utilisation of Bayer red mud. Resour Conserv Recycl 141:483–498
Lyu F, Hu Y, Wang L, Sun W (2021) Dealkalization processes of bauxite residue: a comprehensive review. J Hazard Mater 403:123671
Oprckal P, Mladenovic A, Zupancic N et al (2020) Remediation of contaminated soil by red mud and paper ash. J Clean Prod 256:120440
Samal S (2021) Utilization of red mud as a source for metal ions—a review. Materials 14:2211
Liu WC, Chen XQ, Li W et al (2014) Environmental assessment, management and utilization of red mud in China. J Clean Prod 84:606–610
Li XF, Zhang TA, Lv GZ et al (2023) Summary of research progress on metallurgical utilization technology of red mud. Mineral 13(6):737
Liu X, Han YX, He FY et al (2021) Characteristic, hazard and iron recovery technology of red mud—a critical review. J Hazard Mater 420:126542
Wang SH, Jin HX, Deng Y, Xiao YD (2021) Comprehensive utilization status of red mud in China: a critical review. J Clean Prod 289:125136
Wang K, Dou ZH, Liu Y et al (2022) Summary of research progress on separation and extraction of valuable metals from Bayer red mud. Environ Sci Pollut Res 29:89834–89852
Archambo MS, Kawatra SK (2021) Utilization of bauxite residue: recovering iron values using the iron nugget process. Miner Process Extr Metall Rev 42(4):222–230
Wang K, Liu Y, Dou ZH et al (2022) A novel method of extracting iron from high-iron red mud and preparing low-carbon cement clinker from tailings. JOM 74(7):2750–2759
Li XF, Zhang TA, Lv GZ et al (2023) Study on the application of straw carbon as reductant in the recovery of iron from high-iron red mud. JOM 75(12):5699–5708
Zhu CX, Zhou SW, Wei YG et al (2023) Recovery of fluoride from spent cathode carbon block by combustion combined with water leaching process. J Clean Prod 408:137229
Dong LM, Jiao F, Liu W et al (2023) Harmless recovery and utilization of electrolytic aluminum spent cathode carbon block: a comprehensive review. J Clean Prod 429:139326
Andrade LF, Palmieri MJ, Davide LC (2017) Effects of long exposure to spent potliner on seeds, root tips, and meristematic cells of Allium cepa L. Environ Monitor Assess 189:48910
Sun G, Zhang G, Liu J et al (2019) (Co-)combustion behaviors and products of spent potlining and textile dyeing sludge. J Clean Prod 224:384–395
Sun G, Zhang G, Liu J et al (2021) Thermal behaviors, combustion mechanisms, evolved gasses, and ash analysis of spent potlining for a hazardous waste management. J Environ Sci 107:124–137
Wang Y, Chen X, Zhang S, Yang P (2020) Recycling of spent pot lining first cut from aluminum smelters by utilizing the two-step decomposition characteristics of dolomite. Materials 13:528322
Lu FH, Su XD, Huang F et al (2020) Co-treatment of spent pot-lining and red mud for carbon reutilization and recovery of iron, aluminum and sodium by reductive roasting process. Miner Metals Mater Soc ASM Int 51B:1564
Courbariaux Y, Chaouki J, Guy C (2004) Update on spent potliners treatments: kinetics of cyanides destruction at high temperature. Ind Eng Chem Res 43(18):5828–5837
Silveira BI, Dantas AE, Blasquez JE, Santos RPK (2002) Characterization of inorganic fraction of spent potliners: evaluation of the cyanides and fluorides content. J Hazard Mater 89(2–3):177–183
Yang K, Gong P, Tian Z et al (2020) Recycling spent carbon cathode by a roasting method and its application in Li-ion batteries anodes. J Clean Prod 261:121090
Lisbona DF, Somerfield C, Steel KM (2013) Leaching of spent pot-lining with aluminium nitrate and nitric acid: effect of reaction conditions and thermodynamic modelling of solution speciation. Hydrometallurgy 134:132–143
Birry L, Poirier S (2020) The LCL&L process: a sustainable solution for the treatment and recycling of spent pot lining. Light Metals 2020:1237–1242
Liu GH, Fan KS, Li XB (2006) Sodium aluminosilicate hydrate in alumina production. Light Metals 2:13–17
Li XF, Zhang TA, Wang K et al (2022) Recovery of iron from high-iron Bayer red mud by melting reduction with spent cathode carbon block. Light Metals 2022:56–64
Acknowledgements
This study was funded by the National Natural Science Foundation of China (52204419), the National Natural Science Foundation of Liaoning (2022-BS-076), and the Science and Technology Major Project of Guangxi (2021AA12013). Jiangxi Province ‘Double Thousand Plan’ Innovation Leaders Short-term Project-Natural Science Category (S2021DQKJ2198).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was M. Akbar Rhamdhani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhang, Ta., Lv, G. et al. Waste Treatment by Waste: Study on Iron Recovery from High-Iron Red Mud by Spent Cathode Carbon Block as Reductant and the Behavior of Na/F. J. Sustain. Metall. (2024). https://doi.org/10.1007/s40831-024-00823-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40831-024-00823-4