Abstract
The effect of Ryanodine receptor2 (RyR2) and its stabilizer on cardiac hypertrophy is not well known. C57/BL6 mice underwent transverse aortic contraction (TAC) or sham surgery were administered dantrolene, the RyR2 stabilizer, or control drug. Dantrolene significantly alleviated TAC-induced cardiac hypertrophy in mice, and RNA sequencing was performed implying calcineurin/NFAT3 and TNF-α/NF-κB/NLRP3 as critical signaling pathways. Further expression analysis and Western blot with heart tissue as well as neonatal rat cardiomyocyte (NRCM) model confirmed dantrolene decreases the activation of calcineurin/NFAT3 signaling pathway and TNF-α/NF-κB/NLRP3 signaling pathway, which was similar to FK506 and might be attenuated by calcineurin overexpression. The present study shows for the first time that RyR2 stabilizer dantrolene attenuates cardiac hypertrophy by inhibiting the calcineurin, therefore downregulating the TNF-α/NF-κB/NLRP3 pathway.
Graphical Abstract
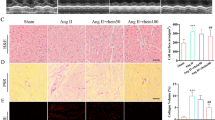
Schematic diagram of the mechanism of Dantrolene. Dantrolene attenuates cardiac hypertrophy through inhibition of TNF-α/NF-κB/NLRP3 pathway by downregulating Calcineurin/NFAT3 pathway.







Similar content being viewed by others
Data Availability
The datasets used and-or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACTA1:
-
Actin, alpha 1
- BW:
-
Body weight
- CH:
-
Cardial hypertrophy
- CPVT:
-
Catecholaminergic polymorphic ventricular tachycardia
- FDR:
-
False discovery rate
- GO:
-
Gene ontology
- HW:
-
Heart weight
- H&E taining:
-
Hematoxylin & Eosin staining
- HDAC:
-
Histone deacetylase
- IκB:
-
Inhibitor of NF-κB
- LVEDP:
-
Left ventricular diastolic pressure
- LVESP:
-
Left ventricular systolic pressure
- LCC:
-
L-type calcium channel
- MH:
-
Malignant hyperthermia
- mTAC:
-
Minimally invasive transverse aortic constriction
- MEF-2:
-
Myocyte enhancer factor-2
- β-MHC:
-
Myosin, heavy polypeptide 7, cardiac muscle, and beta
- NCX:
-
Na+-Ca2+exchanger
- NPPA:
-
Natriuretic peptide type A
- NPPB:
-
Natriuretic peptide type B
- NRCM:
-
Neonatal rat cardiomyocyte
- NF-κB:
-
Nuclear factor kappa-B
- PE:
-
Phenylephrine
- RyR2:
-
Ryanodine receptor 2
- SERCA2:
-
Sarcoplasmic reticulum calcium-transporting ATPases 2
- TL:
-
Tibia length
- TAC:
-
Transverse aortic contraction
- WGA taining:
-
Wheat Germ Agglutinin staining
References
Pandey A, Keshvani N, Ayers C, Correa A, Drazner MH, Lewis A, et al. Association of cardiac injury and malignant left ventricular hypertrophy with risk of heart failure in African Americans: The Jackson heart study. JAMA Cardiol 2019;4(1):51–8. https://doi.org/10.1001/jamacardio.2018.4300.
Jónsdóttir LS, Sigfússon N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovasc Risk. 2002;9(2):67–76.
Kang YJ. Cardiac hypertrophy: a risk factor for QT-prolongation and cardiac sudden death. Toxicol Pathol. 2006;34(1):58–66. https://doi.org/10.1080/01926230500419421.
Messerli FH, Schmieder R. Left ventricular hypertrophy. A cardiovascular risk factor in essential hypertension. Drugs. 1986;31(Suppl 4):192–201. https://doi.org/10.2165/00003495-198600314-00023.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. https://doi.org/10.1038/nrm1983.
Soudani N, Ghantous CM, Farhat Z, Shebaby WN, Zibara K, Zeidan A. Calcineurin/NFAT activation-dependence of leptin synthesis and vascular growth in response to mechanical stretch. Front Physiol. 2016;7:433. https://doi.org/10.3389/fphys.2016.00433.
You J, Wu J, Zhang Q, Ye Y, Wang S, Huang J, et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am J Physiol Heart Circ Physiol. 2018;314(3):H552–62. https://doi.org/10.1152/ajpheart.00212.2017.
Tanaka S, Fujio Y, Nakayama H. Caveolae-specific CaMKII signaling in the regulation of voltage-dependent calcium channel and cardiac hypertrophy. Front Physiol. 2018;9:1081. https://doi.org/10.3389/fphys.2018.01081.
Meissner G. The structural basis of ryanodine receptor ion channel function. J Gen Physiol. 2017;149(12):1065–89. https://doi.org/10.1085/jgp.201711878.
Jayaraman T, Brillantes AM, Timerman AP, Fleischer S, Erdjument-Bromage H, Tempst P, et al. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992;267(14):9474–7.
Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol. 2003;160(6):919–28. https://doi.org/10.1083/jcb.200211012.
Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110(11):1474–83. https://doi.org/10.1161/circresaha.112.268094.
Alsina KM, Hulsurkar M, Brandenburg S, Kownatzki-Danger D, Lenz C, Urlaub H, et al. Loss of protein phosphatase 1 regulatory subunit PPP1R3A promotes atrial fibrillation. Circulation. 2019;140(8):681–93. https://doi.org/10.1161/circulationaha.119.039642.
Zamiri N, Massé S, Ramadeen A, Kusha M, Hu X, Azam MA, et al. Dantrolene improves survival after ventricular fibrillation by mitigating impaired calcium handling in animal models. Circulation. 2014;129(8):875–85. https://doi.org/10.1161/circulationaha.113.005443.
Kaur H, Katyal N, Yelam A, Kumar K, Srivastava H, Govindarajan R. Malignant hyperthermia. Mo Med. 2019;116(2):154–9.
Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene–a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59(4):364–73. https://doi.org/10.1111/j.1365-2044.2004.03658.x.
Shannon TR, Lew WY. Diastolic release of calcium from the sarcoplasmic reticulum: a potential target for treating triggered arrhythmias and heart failure. J Am Coll Cardiol. 2009;53(21):2006–8. https://doi.org/10.1016/j.jacc.2009.02.032.
Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53(21):1993–2005. https://doi.org/10.1016/j.jacc.2009.01.065.
Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123(1):46–52. https://doi.org/10.1172/jci62834.
Kajii T, Kobayashi S, Shiba S, Fujii S, Tamitani M, Kohno M, et al. Dantrolene prevents ventricular tachycardia by stabilizing the ryanodine receptor in pressure- overload induced failing hearts. Biochem Biophys Res Commun. 2020;521(1):57–63. https://doi.org/10.1016/j.bbrc.2019.10.071.
Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol. 2012;302(4):H953–63. https://doi.org/10.1152/ajpheart.00936.2011.
Ohkusa T, Hisamatsu Y, Ueyama T, Kobayashi S, Yano M, Maekawa T, et al. Effects of dantrolene sodium on progression of left ventricular hypertrophy induced by pressure overload in rats. J Cardiovasc Pharmacol. 1998;31(4):520–4. https://doi.org/10.1097/00005344-199804000-00008.
Hamada T, Gangopadhyay JP, Mandl A, Erhardt P, Ikemoto N. Defective regulation of the ryanodine receptor induces hypertrophy in cardiomyocytes. Biochem Biophys Res Commun. 2009;380(3):493–7. https://doi.org/10.1016/j.bbrc.2009.01.152.
Wheatley AM, Butkow N, Grote J, Musiker J, Rosendorff C. The effect of propranolol, verapamil and dantrolene treatment on cardiac hypertrophy, enhanced myocardial contractility and tachycardia in the hyperthyroid rat. Pharmacol Res. 1990;22(3):307–18. https://doi.org/10.1016/1043-6618(90)90728-v.
Wu Z, Yang B, Liu C, Liang G, Eckenhoff MF, Liu W, et al. Long-term dantrolene treatment reduced intraneuronal amyloid in aged Alzheimer triple transgenic mice. Alzheimer Dis Assoc Disord. 2015;29(3):184–91. https://doi.org/10.1097/wad.0000000000000075.
Quinn JL, Huynh T, Uaesoontrachoon K, Tatem K, Phadke A, Van der Meulen JH, et al. Effects of dantrolene therapy on disease phenotype in dystrophin deficient mdx mice. PLoS Curr. 2013;5. https://doi.org/10.1371/currents.md.e246cf493a7edb1669f42fb735936b46.
Wang DW, Mokhonova EI, Kendall GC, Becerra D, Naeini YB, Cantor RM, et al. Repurposing dantrolene for long-term combination therapy to potentiate antisense-mediated DMD exon skipping in the mdx mouse. Mol Ther Nucleic Acids. 2018;11:180–91. https://doi.org/10.1016/j.omtn.2018.02.002.
Dawn B, Guo Y, Rezazadeh A, Huang Y, Stein AB, Hunt G, et al. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98(8):1098–105. https://doi.org/10.1161/01.Res.0000218454.76784.66.
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. https://doi.org/10.1186/gb-2013-14-4-r36.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. https://doi.org/10.1093/bioinformatics/btp616.
Xia H, Chen D, Wu Q, Wu G, Zhou Y, Zhang Y, et al. CELF1 preferentially binds to exon-intron boundary and regulates alternative splicing in HeLa cells. Biochim Biophys Acta Gene Regul Mech. 2017;1860(9):911–21. https://doi.org/10.1016/j.bbagrm.2017.07.004.
Cheng Y, Qin K, Huang N, Zhou Z, Xiong H, Zhao J, et al. Cytokeratin 18 regulates the transcription and alternative splicing of apoptotic-related genes and pathways in HeLa cells. Oncol Rep. 2019;42(1):301–12. https://doi.org/10.3892/or.2019.7166.
Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–84. https://doi.org/10.1016/s0166-4328(01)00297-2.
Peng C, Luo X, Li S, Sun H. Phenylephrine-induced cardiac hypertrophy is attenuated by a histone acetylase inhibitor anacardic acid in mice. Mol Biosyst. 2017;13(4):714–24. https://doi.org/10.1039/c6mb00692b.
Gao S, Liu XP, Wei LH, Lu J, Liu P. Upregulation of α-enolase protects cardiomyocytes from phenylephrine-induced hypertrophy. Can J Physiol Pharmacol. 2018;96(4):352–8. https://doi.org/10.1139/cjpp-2017-0282.
Shaikh F, Bhatt LK. Cardioprotective effect of Polymyxin-B and Dantrolene combination on isoproterenol-induced hypertrophic cardiomyopathy in rats, via attenuation of Calmodulin-dependent protein kinase II. Chem Biodivers. 2022. https://doi.org/10.1002/cbdv.202200309.
Kohno M, Kobayashi S, Yamamoto T, Yoshitomi R, Kajii T, Fujii S, et al. Enhancing calmodulin binding to cardiac ryanodine receptor completely inhibits pressure-overload induced hypertrophic signaling. Commun Biol. 2020;3(1):714. https://doi.org/10.1038/s42003-020-01443-w.
deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010;(38):1729. https://doi.org/10.3791/1729.
Zaw AM, Williams CM, Law HK, Chow BK. Minimally invasive transverse aortic constriction in mice. J Vis Exp 2017;(121):55293. https://doi.org/10.3791/55293.
Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322(4):1178–91. https://doi.org/10.1016/j.bbrc.2004.07.121.
Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51(4):468–73. https://doi.org/10.1016/j.yjmcc.2011.01.012.
Liu J, Zhao Z, Wen J, Wang Y, Zhao M, Peng L, et al. TNF-α differently regulates TRPV2 and TRPV4 channels in human dental pulp cells. Int Endod J. 2019;52(11):1617–28. https://doi.org/10.1111/iej.13174.
Varfolomeev E, Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine. 2018;101:26–32. https://doi.org/10.1016/j.cyto.2016.08.035.
Afonina IS, Zhong Z, Karin M, Beyaert R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol. 2017;18(8):861–9. https://doi.org/10.1038/ni.3772.
An Y, Zhang H, Wang C, Jiao F, Xu H, Wang X, et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. Faseb j. 2019;33(11):12515–27. https://doi.org/10.1096/fj.201802805RR.
Haskó G, Szabó C, Németh ZH, Lendvai B, Vizi ES. Modulation by dantrolene of endotoxin-induced interleukin-10, tumour necrosis factor-alpha and nitric oxide production in vivo and in vitro. Br J Pharmacol. 1998;124(6):1099–106. https://doi.org/10.1038/sj.bjp.0701934.PubMedPMID:9720779;PubMedCentralPMCID:PMCPMC1565490.
Abdullah HI, Pedraza PL, Hao S, Rodland KD, McGiff JC, Ferreri NR. NFAT regulates calcium-sensing receptor-mediated TNF production. Am J Physiol Renal Physiol. 2006;290(5):F1110–7. https://doi.org/10.1152/ajprenal.00223.2005.
Liu FX, Wu CL, Zhu ZA, Li MQ, Mao YQ, Liu M, et al. Calcineurin/NFAT pathway mediates wear particle-induced TNF-α release and osteoclastogenesis from mice bone marrow macrophages in vitro. Acta Pharmacol Sin. 2013;34(11):1457–66. https://doi.org/10.1038/aps.2013.99.
Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem. 2004;279(43):44573–81. https://doi.org/10.1074/jbc.M404175200.
Parra V, Rothermel BA. Calcineurin signaling in the heart: The importance of time and place. J Mol Cell Cardiol. 2017;103:121–36. https://doi.org/10.1016/j.yjmcc.2016.12.006.
Acknowledgements
This work was supported by the fund of Natural Science Foundation of China (grant number 81970209). Thanks are also due to Professor Nanthakumar for the valuable discussion.
Funding
This study was supported by the fund of Natural Science Foundation of China (grant number 81970209).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Statement
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees. No human studies were carried out by the authors for this article.
Conflict of Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplemental Figure 1.
The expression of eight DEGs in the Treated and Untreated TAC group. The left 2 bars of each figure show the relative gene expression detected by transcriptome sequencing. The right two bars show the gene expression of the same gene verified by qRT-PCR. *P < 0.05, **P < 0.01, ***P < 0.001. (PNG 591 kb)

Supplemental Figure 2.
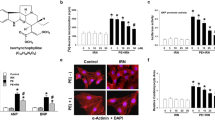
Dose screening test of PE and dantrolene in NRCMs. Cardiomyocytes were treated with various concentrations of PE (0, 1, 10, 50, 100, and 500 μM) for 24 h. (A) The cell viability decreased with an increase in the concentration of PE. B. The expression of hypertrophy markers increased with an increase in PE concentration. (C-E) The PE intervention myocardial hypertrophy group was treated with 50 μM PE. In addition to PE, the dantrolene intervention group was treated with 0, 1, 10, 50, 100, and 500 μM of dantrolene for 24 h. Cell viability (C) and quantitative protein for hypertrophy marker BNP (D, E) were detected to determine the dantrolene concentration. *compared with the control group, P < 0.05, **compared with the control group, P < 0.01, ***compared with the control group, P < 0.001. #compared with the PE group, P < 0.05,##compared with the control group, P < 0.01, ### compared with the control group, P < 0.001. (PNG 458 kb)

Supplemental Figure 3.
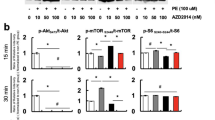
The effects of dantrolene on PE-induced hypertrophy in NRCMs. The changes in protein expression related to calcineurin/NFAT3 pathways in NRCMs under the effect of dantrolene, FK506 or CnA overexpression. **compared with the control group, P < 0.01, ***compared with the control group, P < 0.001. ## compared with the PE control group, P < 0.01, ###compared with the PE control group, P < 0.001. △△compared with the PE + dantrolene group, P < 0.01, △△△compared with the PE+ dantrolene group, P < 0.001. (PNG 484 kb)
Supplemental Table 1
(DOCX 29 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Li, S., Liu, X. et al. RyR2 Stabilizer Attenuates Cardiac Hypertrophy by Downregulating TNF-α/NF-κB/NLRP3 Signaling Pathway through Inhibiting Calcineurin. J. of Cardiovasc. Trans. Res. (2024). https://doi.org/10.1007/s12265-023-10376-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12265-023-10376-8