Abstract
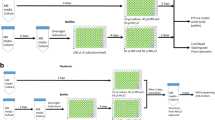
lWater samples were collected from a tailings pond wastewater site in the Rudnik mountain area, where polymetallic ore flotation processes generate high concentrations of Pb2+, Zn2+, and Cu2+. The study aimed to identify microorganisms resistant to heavy metals and assess their potential for bioremediation. Growth capabilities under varying conditions, including temperature, pH, and NaCl concentrations, were analyzed using a spectrophotometer. Minimal inhibitory and lethal concentrations of tested substances were determined for both planktonic cells and their biofilms. Key isolates, namely Bacillus altitudinis PMFKG-R3, B. pumilus PMFKG-R15, B. cereus PMFKG-R46, Pseudomonas veronii PMFKG-R30, and Pantoea agglomerans PMFKG-R20, demonstrated growth ability at both 22 and 37°C and exhibited halotolerance, albeit sensitivity to acidic pH. Most isolates in both planktonic and biofilm forms displayed notable resistance to heavy metals, particularly Pb2+ and Zn2+, in line with the sampling location. Notably, planktonic cells were sensitive to antibiotics, while biofilms exhibited slightly higher resistance. Promising candidates for bioremediation purposes were identified in P. veronii PMFKG-R30 and P. agglomerans PMFKG-R20, which displayed resistance to heavy metals and sensitivity to antibiotics.




Similar content being viewed by others
REFERENCES
Bacterial Stress Responses, Storz, G. and Hengge-Aronis R., Eds., Washington, D.C.: ASM Press, 2000, pp. 161–178.
Kang, C.H., Kwon, Y.J., and So, J.S., Ecol. Eng., 2016, vol. 89, pp. 64–69. https://doi.org/10.1016/j.ecoleng.2016.01.023
Igiri, B.E., Okoduwa, S.I.R., Idoko, G.O., Akabuogu, E.P., Adeyi A.O., and Ejiogu, I.K., J. Toxicol., 2018, vol. 2018, p. 2568038. https://doi.org/10.1155/2018/2568038
Rajbanshi, A., Our Nature, 2008, vol. 6, no. 1, pp. 52–57.
Approaches in Bioremediation. The New Era of Environmental Microbiology and Nanobiotechnology, Prasad R. and Aranda E., Eds., Springer International Publishing, 2018, pp. 1–28. https://doi.org/springer.com/de/book/9783030023683
Edwards, S.J. and Kjellerup, B.V., Appl. Microbiol. Biotechnol., 2013, vol. 97, no. 23, pp. 9909–9921. https://doi.org/10.1007/s00253-013-5216-z
Gupta Mahendra, K., Kiran, K., Amita, S., and Shikha, G., J. Environ. Res. Dev., 2014, vol. 8, no. 4, pp. 883–889.
Narasimhulu, K., Rao, P.S., and Vinod, A.V., J. Microbial Biochem. Technol., 2010. vol. 2, no. 3, pp. 74–76.
Mladenović, K.G., Muruzović, M.Ž., Žugić-Petrović, T.D., and Čomić, L.R., Kragujev. J. Sci., 2018, vol. 40, pp. 205–216. https://doi.org/10.5937/KgJSci1840205M
Grujić, S., Vasić, S., Čomić, L., Ostojić, A., and Radojević, I., Water Sci. Technol., 2017, vol. 76, no. 4, pp. 806–812. https://doi.org/10.2166/wst.2017.248
Shylla, L., Barik, S.K., and Joshi, S.R., Arch. Microbiol., 2021, vol. 203, no. 5, pp. 2379–2392. https://doi.org/10.1007/s00203-021-02218-5
Branković, S., Bugarčić, M., Bugarčić, F.Ž., Ostojić, A., Petronijević J., Rosić, G., et al., Environ. Sci. Pollut. Res., 2022, vol. 29, no. 3, pp. 1–13. https://doi.org/10.1007/s11356-022-19986-2
Methods for General and Molecular Microbiology, Reddy, C.A., Beveridge, T.J., Breznak, J.A., Marzluf, G.A., Schmidt, T.M., and Snyder, L.R. Eds., Wiley Online Library, 2007, 3rd ed. https://doi.org/10. 1128/9781555817497
O'Toole, G.A. and Kolter, R., Mol. Microbiol., 1998, vol. 28, no. 3, pp. 449–461. https://doi.org/10.1046/j.1365-2958.1998.00797.x
Muruzović, M.Ž., Mladenović, K.G., Stefanović, O.D., Vasić, S.M., and Čomić, L.R., J. Food Drug Anal., 2016, vol. 24, no. 3, pp. 539–547. https://doi.org/10.1016/j.jfda.2016.02.007
Andrews, J.M., J. Antimicrob. Chemother., 2005. vol. 56, pp. 60–76.
Sarker, S.D, Nahar, L., and Kumarasamy, Y., Methods, 2007, vol. 42, no. 4, pp. 321–324. https://doi.org/10.1016/j.ymeth.2007.01.006
Padan, E., Bibi, E., Ito, M., and Krulwich, T.A., Biochim. Biophys. Acta Biomembr., 2005, vol. 1717, no. 2, pp. 67–88. https://doi.org/10.1016/j.bbamem.2005.09.010
Hahne, H., Mäder, U., Otto, A., Bonn, F., Steil, L., Bremer, E., et al., J. Bacteriol., 2010. vol. 192, no. 3, pp. 870–882. https://doi.org/10.1128/jb.01106-09
Kumar, G.P., Mir, Ahmed, S.K.M.H., Desai, S., Amalraj, E.L.D., and Rasul, A., Int. J. Bacteriol., 2014, vol. 2014, p. 195946. https://doi.org/10.1155/2014/195946
Han, L.J., Li, J.S., Xue, Q., and Wang, M.Q., Earth Environ. Sci., 2021, vol. 861, no. 7, p. 072020. https://doi.org/10.1088/1755-1315/861/7/072020
Hong, Z., Rong, X., Cai, P., Liang, W., and Huang Q., Geomicrobiol. J., 2011, vol. 28, no. 8, pp. 686–691. https://doi.org/10.1080/01490451.2010.514025
Sharma, D., Misba, L., and Khan, A.U., Antimicrob. Resist. Infect. Control, 2019, vol. 8, no. 1, pp. 1–10. https://doi.org/10.1186/s13756-019-0533-3
Ikhwani, A.Z.N., Nurlaila, H.S., Ferdinand, F.D.K., Fachria, R., Hasan A.E.Z., Yani M., et al., Earth Environ. Sci., 2017. vol. 58, no. 1, p. 012056. https://doi.org/10.1088/1755-1315/58/1/012056
Ashwitha, K., Rangeshwaran, R., Vajid, N.V., Sivakumar, G., Jalali, S.K., Rajalaksmi, K., et al., J. Biol. Control, 2013, vol. 27, no. 4, pp. 319–328.
Busnelli, M.P., Lazzarini Behrmann, I.C., Ferreira, M.L., Candal, R.J., Ramirez, S.A., and Vullo, D.L., Front. Microbiol., 2021, vol. 12, p. 622600. https://doi.org/10.3389/fmicb.2021.622600
Mèndez, N., Ramìrez, S.A.M., Cerettia, H.M., Zalts, A., Candal, R., and Vullo, D.L., Global J. Environ. Sci. Technol., 2011, vol. 1, no. 3.
González, C., Daniel, J., Barrera, R., Ángel, M., Ramírez, R., Sánchez, Y.A., et al., Rev. Mexicana Cienc. Agríc., 2016, vol. 7, no. 4, pp. 961–968.
Neeta, B., Maansi, V., and Harpreet, S.B., Afr. J. Microbiol. Res., 2016, vol. 10, no. 5, pp. 127–137. https://doi.org/10.5897/AJMR2015.7769
AbdAlhussen, L.S. and Darweesh M.F., Int. J. Chemtech. Res., 2016, vol. 9, no. 8, pp. 430–437.
Zou, H.Y., He, L.Y., Gao, F.Z., Zhang, M., Chen, S., Wu, D.L., et al., Sci. Total Environ., 2021, vol. 772, p. 145516. https://doi.org/10.1016/j.scitotenv.2021.145516
Timoney, J.F., Port, J., Giles, J., and Spanier, J., Appl. Environ. Microbiol., 1978, vol. 36, no. 3, pp. 465–472. https://doi.org/10.1128/aem.36.3.465-472.1978
Shammi, T. and Ahmed, S., Bangladesh J. Microbiol., 2013. vol. 30, nos. 1–2, pp. 17–22. https://doi.org/10.3329/bjm.v30i1-2.28448
Choudhury, P. and Kumar, R., Ind. J. Med. Res., 1996, vol. 104, pp. 148–151.
Teixeira, P., Tacão, M., Alves, A., and Henriques, I., Mar. Pollut. Bull., 2016, vol. 110, no. 1, pp. 75–81. https://doi.org/. 2016.06.086https://doi.org/10.1016/j.marpolbul
Funding
This work was supported by the Serbian Ministry of Education, Science and Technological Development (Agreement no. 451-03-68/2022-14/200122), Multilateral scientific and technological cooperation in the Danube region for 2020-2022. year (DS10) 337-00-00322/2019-09/107 Metal microorganism’s interaction as a basis for progressive biotechnological processes and COST Action 18113 (STSM grant ECOSTSTSM-Request-CA18113-45768) EuroMicropH—Understanding and exploiting the impacts of low pH on micro-organisms.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Fig. S1 . MALDI-TOF MS spectra of isolate B. altitudinis PMFKG-R3; the absolute intensities of the ions are shown on the y axis, and the masses (m/z) of the ions are shown on the x-axis.
Fig. S2 . MALDI-TOF MS spectra of isolate B. pumilus PMFKG-R15; the absolute intensities of the ions are shown on the y axis, and the masses (m/z) of the ions are shown on the x-axis.
Fig. S3 . MALDI-TOF MS spectra of isolate B. cereus PMFKG-R46; the absolute intensities of the ions are shown on the y axis, and the masses (m/z) of the ions are shown on the x-axis.
Fig. S4 . MALDI-TOF MS spectra of isolate P. veronii PMFKG-R30; the absolute intensities of the ions are shown on the y axis, and the masses (m/z) of the ions are shown on the x-axis.
Fig. S5 . MALDI-TOF MS spectra of isolate P. agglomerans PMFKG-R20; the absolute intensities of the ions are shown on the y axis, and the masses (m/z) of the ions are shown on the x-axis.
Rights and permissions
About this article
Cite this article
Radojević, I.D., Ćirković, K.G., Grujović, M.Ž. et al. Characterization of Bacterial Isolates from Tailings Pond and Their Resistance to Heavy Metals and Antibiotics. Appl Biochem Microbiol 60, 347–357 (2024). https://doi.org/10.1134/S0003683824020157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683824020157