Abstract
This study is the first investigation for using sex-related gene expression in tail fin tissues of seabass as early sex determination without killing the fish. The European seabass (Dicentrarchus labrax) is gonochoristic and lacks distinguishable sex chromosomes, so, sex determination is referred to molecular actions for some sex-related genes on autosomal chromosomes which are well known such as cyp19a1a, dmrt1a, and dmrt1b genes which play crucial role in gonads development and sex differentiation. cyp19a1a is expressed highly in females for ovarian development and dmrt1a and dmrt1b are for testis development in males. In this study, we evaluated the difference in the gene expression levels of studied genes by qPCR in tail fins and gonads. We then performed discriminant analysis (DA) using morphometric traits and studied gene expression parameters as predictor tools for fish sex. The results revealed that cyp19a1a gene expression was significantly higher in future females’ gonads and tail fins (p ≥ 0.05). Statistically, cyp19a1a gene expression was the best parameter to discriminate sex even the hit rate of any other variable by itself could not correctly classify 100% of the fish sex except when it was used in combination with cyp19a1a. In contrast, Dmrt1a gene expression was higher in males than females but there were difficulties in analyzing dmrt1a and dmrt1b expressions in the tail because levels were low. So, it could be used in future research to differentiate and determine the sex of adult fish using the cyp19a1a gene expression marker without killing or sacrificing fish.
Similar content being viewed by others
Introduction
Aquaculture has made a significant contribution to global food production, particularly in providing a sustainable source of animal proteins. According to the Food and Agriculture Organization (FAO 2019), the total aquaculture production worldwide reached approximately 85.4 million tons. In Egypt, which boasts the largest aquaculture industry in Africa, aquaculture plays a pivotal role in the provision of fish, accounting for a total production of around 1.8 million tons; however, 11.52% of the total production is attributed to the European seabass (Dicentrarchus labrax), which is the first marine species cultivated (Kaleem and Bio Singou Sabi 2021).
The European seabass (D. labrax) is a member of the Moronidae family of Teleosts, which is closely related to the Serranidae (groupers) family and includes numerous hermaphroditic species. It is worth noting that D. labrax is gonochoristic and lacks readily distinguishable morphological secondary sexual features or sex chromosomes (Piferrer et al. 2005; Vandeputte et al. 2007). The initial histological signs of ovarian and testicular differentiation become evident when male and female fish attain a standard length (SL) of approximately 79–90 mm and 83–95 mm, respectively (occurring around 150–200 days post fertilization (dpf)) (Saillant et al. 2003). Economically, the aquaculture sector is interested in the monosex breeding of female cohorts because females take longer to mature and grow than males, which results in more weight gain (Wootton and Smith 2014). Hence, investigations for the identification of sex-determining genes and the elucidation of their functioning in fish are expected to provide a conceptual framework to inform the development of strategies for sex control in breeding programs (Long et al. 2020; Chen et al. 2022).
Genes responsible for sex determination in various fish species reveal a wide spectrum of diversity. So, understanding the mechanisms behind sex determination in the majority of fish species used in aquaculture is challenging due to the intricate and varied nature of these processes (Lin et al. 2017). In the case of seabass, several genes involved in sex determination and differentiation have been identified and their expression profiles have been examined. These genes include cyp19a1a (Dalla Valle et al. 2002; Blázquez et al. 2008), cyp19a1b (Blázquez and Piferrer 2004; Blázquez et al. 2008), amh (Halm et al. 2007), dax1 (Martins et al. 2007), estrogen receptors (ers) (Halm et al. 2004; Blázquez et al. 2008), androgen receptor b (arb), (Blázquez and Piferrer 2005), and cyp11b (Socorro et al. 2007).
The cytochrome P450 family 19 subfamily A (cyp19a), also referred to as cyp19a1, cyp19a1a, and ovarian aromatase (Guiguen et al. 2010), demonstrates a distinct pattern of expression. Where, cyp19a1a exhibits a high level of expression in the ovary, with comparatively lower expression in the testis and brain (Dalla Valle et al. 2002). Aromatase, well known for its pivotal role in sex differentiation in fish, is presumed to play a significant role in the process of sex determination in seabass (Blázquez and Piferrer 2004). In this context, research by Blázquez et al. (2009) has demonstrated that cyp19a1a expression is significantly elevated in individuals destined to become females, establishing it as a robust molecular marker for predicting future ovarian differentiation in seabass.
The double sex and mab-3-related transcription factor 1 (Dmrt1) gene belongs to a gene family characterized by a zinc-finger-like DNA-binding motif known as the DM domain which was identified in both invertebrate and vertebrate species (Guo et al. 2005). Dmrt1 is notably expressed in the gonads, especially in the testes, of various species such as tilapia (Oreochromis niloticus), rainbow trout (Onchorynchus mykiss), medaka (Oryzias latipes), and fugu (Takifugu rubripes) (Guan et al. 2000; Marchand et al. 2000; Brunner et al. 2001; Yamaguchi et al. 2006). In the case of the European sea bass (D. labrax), a single gene encodes two distinct dmrt1 transcripts, dmrt1a and dmrt1b, both of which are specifically expressed in males (Deloffre et al. 2009).
Selective breeding programs depend on the identification of secondary sexual features or identifiable sex chromosomes which are lacking in European seabass, although identified morphological features are a crucial prerequisite to achieving results for breeding programs as well as for safeguarding biodiversity, so using cyp19a1a, dmrt1a, and dmrt1b expressions in sexually differentiated adult fish have already been shown to be sexually dimorphic in several investigations and their expression levels might potentially be a good indicator of the phenotypic sex. So, the main objective of this study is to investigate the expression of cyp19a1a, dmrt1a, and dmrt1b genes in gonads of 1.5-year-old sexually differentiated seabass males and females in addition to their expression in tail fins (caudal) tissues to assess their expression as molecular markers could be used in future research to differentiate and determine the sex of adult fish without kill or sacrificing fish.
Materials and Methods
Sample Collection
The experimental fish used in the present study were 24 adult fish (12 males and 12 females; age 1.5 years, after the gonadal developmental period (540 days post fertilization, dpf)); individuals were collected from the fish hatchery Kilo 21, Alexandria, Egypt. In summary, during the sampling process, the fish were assessed for various parameters, including body weight (BW), total length (TL), and standard length (SL). They were then sorted into two distinct groups based on size: one group comprised the smaller fish with an average SL of 18.5 cm (referred to as the male-dominant group), and the other group consisted of larger fish with an average SL of 20.2 cm (referred to as the female-dominant group). This classification was made considering the well-established association in seabass between somatic growth and phenotypic sex from the early stages of development (Vandeputte et al. 2007). Subsequently, the fish gonads and tail or caudal fins from each individual were carefully dissected. The gonads were examined by the microscope to determine their respective sexes. After this, the collected tissues were individually separated in sterilized tube and quickly frozen in liquid nitrogen and stored at – 80 °C for later analysis. All animal maintenance and handling procedures followed the recommendations of the Institutional Animal Care Use Committee, Alexandria University, Egypt (Alex-IACUC) review report AU: 14/20/11/01/2/9.
Fulton’s Condition Factor (K)
The Fulton’s condition factor (K) serves as an indicator of fish well-being and offers valuable insights into aspects such as growth, age, reproductive status, nutritional health, and overall welfare. This factor is determined through the following formula: K = (100 × BW)/TL3, where BW represents body weight (in grams) and TL corresponds to total length (in centimeters) (Gonzalez-Martinez et al. 2021).
RNA Extraction
Total RNA was isolated from the ovary, testis, and apical parts of tail fin clip tissues using the Genozol Tri RNA Kit (Geneaid) according to the manufacturer protocol. The RNA yield quality and concentrations were checked by Nanodrop spectrophotometer (BioDrop, England). The normalization of RNA sample concentration was performed to be 50 ng for each sample.
RT-PCR Reaction for Genes of Interest
Topreal™ One-step RT q-PCR Kit (SYBER Green with low ROX) (enzynomics) is used to perform RT and q-PCR reactions according to the manufacturer procedure for expression analysis. 18s rRNA gene is used as a reference gene. The reaction conditions were as follows: holding at 45 °C for 30 min, PCR reaction was initiated at 95 °C for 10 min, followed by 95 °C for 5 s then annealing step for 30 s for various primers cyp19a1a, dmrt1a, dmrt1b, and 18s rRNA genes. The reaction was repeated for 50 cycles (Table 1). The specificity of the real-time PCR amplification was confirmed through a melting curve analysis, which confirmed that only a single PCR product of the intended size was selectively amplified. The cycle threshold (Ct) was determined for each individual replicate, and the ultimate values were derived from the average of two replicates for each sample. To normalize the expression values of the genes under investigation, the expression values of the reference gene 18s rRNA were used.
The analysis of gene expression for the genes under examination was conducted using the \(2^{-\Delta\Delta {C_t}}\) method, as outlined in the following equation (Livak and Schmittgen 2001):
-
Fold difference = \(2^{-\Delta\Delta {C_t}}\)
-
ΔCt sample – Δ Ct calibrator = ΔΔ Ct
-
Ct GOI s − Ct norm s = Δ Ct sample
-
Ct GOI c − Ct norm c = Δ Ct calibrator
The ΔΔCt method stands as a widely adopted approach for comparing outcomes between experimental samples and employs both a calibrator (e.g., an untreated or wild-type sample) and a normalizer (e.g., the expression of a housekeeping gene). In this method, the Ct values for the gene of interest (GOI) in both the test sample(s) and the calibrator sample are normalized in reference to the Ct value of a normalizer (norm) gene obtained from the same two samples. The resulting ΔΔCt value is then utilized to ascertain the fold difference in gene expression.
Statistical Analysis
Numerous prior studies have already established the sexually dimorphic nature of cyp19a1a gene expression in sexually differentiated juvenile and adult fish. Furthermore, various other genes exhibit differing levels of sex-related disparities in their expression, suggesting their potential as reliable indicators of phenotypic sex. However, it was imperative to validate this hypothesis. To this end, our initial examination focused on the expression of cyp19a1a, dmrt1a, and dmrt1b in the gonads of sexually differentiated sea bass at the age of 1.5 years, with their phenotypic sex determined through histological analysis. Subsequently, we assessed sex-related variations in the expression of cyp19a1a, dmrt1a, and dmrt1b in the tail fins of the same fish sample. We employed a randomized completely block design (RCBD) two-way analysis of variance (ANOVA) to evaluate differences in the mean gene expression among sexually differentiated fish, specifically males and females, using the model as described in CoStat (2017):
where Yijl, observation of the ijlth parameter measured; µ, overall mean; Gi, effect of the ith gene; Sj, effect of jth sex; (GS)ij, interaction genes by sex; eijl, random error. Significant differences (P ≤ 0.05) among means were tested by the method of Duncan (1955). The morphometric characteristics were analyzed by T-test.
Furthermore, the final dataset underwent analysis through discriminant analysis (DA). DA is a statistical method that classifies a set of observations into two categories, based on the values of independent continuous categorical variables or predictors, with the categorical variable (sex, male or female in this context) serving as the grouping variable. The outcome of this analysis generates a linear discriminant function, which allocates values to the categorical variable based on the values of the predictor variables (Legendre and Legendre 2012). A discriminant score can be calculated by considering the weighted combination of the predictor variables:
which Di represents the anticipated score (discriminant score), x stands for the predictor, and b denotes the discriminant coefficient.
Results
Morphometric Characteristics
Total length (TL) and standard length (SL) were significantly different (p ≤ 0.05) between females and males where, the mean values between 22.18 ± 0.24 cm, 20.6 ± 0.23 cm, and 20.22 ± 0.32 cm, and 18.43 ± 0.24 cm, respectively. As for body weight (BW) and Fulton’s condition factor (K) were no significant differences (p ≤ 0.05) between females and males (Table 2).
Expression Profile of (cyp19a, dmrt1a, and dmrt1b) Genes
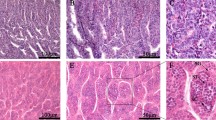
Comparative gene expression analysis of sex determination genes was conducted in tissues of the tail fins as well as the gonads of female and male individuals, investigating that the female ovaries had higher levels of cyp19a1a gene expression than the male’s testis. However, in Dmrt1a and Dmrt1b the gene expression in the male’s testis was higher than female’s ovaries Fig. 1(A–C). The same pattern was detected in tail fin tissues, where cyp19a1a gene expression was higher in the female’s tail fins than the males. Additionally, Dmrt1a gene expression was higher in males than in females Fig. 1(D–F).
Sex-Related Differences in Gene Expression of Key Genes in Gonads Tissues
The expression of cyp19a1a, dmrt1a, and dmrt1b in gonads of 1.5-year-old sexually differentiated females and males’ sea bass fish. Analysis of variance II showed significant differences (p < 0.05) between gene expression, while between sexes, these differences were insignificant. As shown in Table 3, cyp19a1a recorded the highest expression value compared to dmrt1a and dmrt1b genes. Concerning the effect of sex on gene expression, the results reported that there was no significant difference between males and females. Regarding the interaction between G × S, the results reported that cyp19a1a in females’ ovaries recorded a higher value of gene expression than the other interactions.
Sex-Related Differences in Gene Expression of Key Genes in Tail Fin Tissues
The sex-related differences in gene expression of cyp19a1a, dmrt1a, and dmrt1b in tail fins of the same fish samples reported significant differences (p < 0.05) between genes and between sex, where cyp19a1a was the highest expression value. In terms of sex, females were higher than males in gene expression values. Concerning the interaction between gene and sex, cyp19a1a in females’ tail fins had the highest gene expression value among other interactions, as shown in Table 3.
Define Sex Predictors by Discriminant Analysis (DA)
Discriminant analysis (DA) was obtained to determine which variables including morphometric characteristics (TL, SL, K factor, and body weight (BW)) and all studied genes expression in gonads and tail fins were the best to define sex (males and females).
The DA conducted on morphometric characteristics indicated that TL and SL factors showed significant (p ≤ 0.05) indicators for sex differentiation, with F-values of 23.011 and 19.870, and Wilks’ λ test values of 0.303 and 0.335, enabling a 100% correct classification of fish in both cases. Still, body weight and K factor had no significant and high value of Wilks’ λ = 1 and 0.68, respectively, as shown in Table 4.
The expression levels of all studied genes as determined by real-time RT-PCR on samplings from gonads were used to perform the DA. The findings indicate that cyp19a1a expression levels played a significant role (p ≤ 0.05) in sex differentiation, with an F-value of 6.382 and a Wilks’ λ test value of 0.610, correctly classifying 75.0% of the fish sex. In contrast, dmrt1a and dmrt1b did not exhibit significant effects, as indicated by the high value of Wilks’ λ = 0.9 (Table 5). While none of the three genes individually achieved a 100% correct classification of fish sex, their collective use with cyp19a1a improved classification rates. This underscores the significant role of cyp19a1a in discriminating sex in sexually differentiated sea bass.
Subsequently, when considering the expression levels of studied genes from tail fin tissues, the results indicated that cyp19a1a and dmrt1a expression levels significantly (p ≤ 0.05) could indicate fish sex, as evidenced by Wilks’ λ test values of 0.164 and 0.465, respectively. In contrast, dmrt1b did not exhibit significance, displaying a high Wilks’ λ value of 0.835. Notably, cyp19a1a emerged as the most influential variable for discriminating between sexes, with a Wilks’ λ test value of 0.164, successfully classifying 91.7% of the fish (Table 6). Wilks’ λ values range from 0 to 1.0, with smaller λ values indicating better discrimination among groups. As anticipated, no single variable achieved a 100% correct classification of fish on its own, but when used in conjunction with cyp19a1a, classification rates improved. This statistical analysis supports the conclusion that cyp19a1a is the most effective indicator for sex determination in sexually differentiated sea bass.
Discussion
According to size categorizations, Blazquez et al. (1999) have substantiated that female seabass exhibit faster growth rates than their male counterparts right from a young age. This suggests that size can effectively serve as a reliable indicator for sex differentiation in this species. Consequently, employing size grading as a methodology allows us to attribute variations in gene expression and activity to a specific sex during the process of sex differentiation. Our findings are in line with this notion, as they reveal that both total length (TL) and standard length (SL) exhibit significantly greater differences between females and males, further supporting the utility of size-based sex selection in seabass.
In the current study, the expression of the cyp19a1a gene is notably higher in female ovaries compared to other studied genes and aligning with subsequent studies on species such as Southern flounder (Luckenbach et al. 2005), Atlantic halibut (Matsuoka et al. 2006), and rainbow trout (Vizziano et al. 2007). These studies have collectively suggested that cyp19a1a gene expression serves as an early indicator of sex differentiation in these species. Additionally, cyp19a1a is considered a suitable molecular marker for ovarian differentiation in seabass fish (Piferrer and Guiguen 2008; Blázquez et al. 2008).
The identification of gene expression for all three genes (cyp19a1a, dmrt1a, and dmrt1b) in female and male tail fins, our results indicate that cyp19a1a gene expression is the highest in female tail fin tissues, These results may along with a similar scenario in zebrafish which has been documented for SRY HMG box-related gene 9a (Sox9a) expression pattern in testis and pectoral fin (Hofsten and Olsson 2005) and with Panagiotopoulou et al. (2023) who found genetic sex markers in Siberian (Acipenser baerii) and Atlantic (A. oxyrinchus) sturgeons; however, they used a different technique using genetic material extracted from female and male tail fin tissues.
The discriminant analysis finds applications across various research domains, so it is employed in numerical ecology within the context of fisheries management to classify ecosystem exploitation (Tudela et al. 2005). In microbiology, it serves the purpose of discerning the origins of contamination in surface waters, such as whether they stem from human or animal sources (Kaneene et al. 2007). Within the field of forensics, it plays a pivotal role in approximating the gender of unidentified skeletal remains (Kemkes-Grottenthaler 2005). Moreover, discriminant analysis finds applications in medical research, particularly in the differentiation of various types of anemia (Ahluwalia et al. 1995). Notably, it was also employed for the first time to investigate the process of sex differentiation in seabass (Blázquez et al. 2009).
In this study, discriminant analysis was used first to define which variables among morphometric parameters and genes (cyp19a1a, dmrt1a, and dmrt1b) can discriminate fish sex in adult seabass gonads and tail fins. According to the morphometric results, the SL, TL, and K factors can correctly classify 100%, 100%, and 91.7% of fish, respectively. This discovery aligns with the established notion that size serves as a reliable marker for sex selection in seabass, as documented by Blazquez et al. (1999). Furthermore, Blázquez et al. (2009) observed that standard length (SL) could accurately classify 75.7% of fish at 330 days post-fertilization (dpf), and this determination was validated through histological examination of gonadal sex.
Based on the results obtained from the gonadal genes, cyp19a1a emerged as the most effective predictor for sex differentiation (F = 6.382; Wilks’ λ test values = 0.610), accurately classifying 75% of the fish on its own. Furthermore, when cyp19a1a was combined with other genes (G1, G2, and G3), this combination yielded the highest accuracy, correctly identifying 91.7% of the fish. Additionally, when we applied and validated the discriminant analysis to the same genes in tail fins, our findings consistently showed that cyp19a1a alone was the most influential variable in discriminating between sexes (Wilks’ λ test = 0.164), accurately classifying 91.7% of the fish. In this case, any combination of variables achieved a 100% accuracy rate. These results corroborate the research conducted by Blázquez et al. (2009), who reported that the cyp19a1a gene could perfectly classify 100% of the fish at 330 days post-fertilization (dpf).
Conclusion
In this work, we concluded that cyp19a1a can be used as a genetic marker to discriminate between the fish sexes and as an indicator for ovarian differentiation in sexually differentiated seabass. Additionally, the results reported that tissues from the females’ tail fins had significant levels of cyp19a1a gene expression. Therefore, they could be utilized in future studies to distinguish between various adult fish and to identify their sex using molecular markers without killing or sacrificing fish, so they could be used in breeding programs of fish stocks.
Data Availability
No datasets were generated or analysed during the current study.
References
Ahluwalia N, Lammi-Keefe CJ, Bendel RB et al (1995) Iron deficiency and anemia of chronic disease in elderly women: a discriminant-analysis approach for differentiation. Am J Clin Nutr 61:590–596
Blazquez M, Carrillo M, Zanuy S, Piferrer F (1999) Sex ratios in offspring of sex-reversed sea bass and the relationship between growth and phenotypic sex differentiation. J Fish Biol 55:916–930
Blázquez M, González A, Papadaki M et al (2008) Sex-related changes in estrogen receptors and aromatase gene expression and enzymatic activity during early development and sex differentiation in the European sea bass (Dicentrarchus labrax). Gen Comp Endocrinol 158:95–101
Blázquez M, Navarro-Martín L, Piferrer F (2009) Expression profiles of sex differentiation-related genes during ontogenesis in the european sea bass acclimated to two different temperatures. J Exp Zool Part B Mol Dev Evol 312:686–700
Blázquez M, Piferrer F (2004) Cloning, sequence analysis, tissue distribution, and sex-specific expression of the neural form of P450 aromatase in juvenile sea bass (Dicentrarchus labrax). Mol Cell Endocrinol 219:83–94
Blázquez M, Piferrer F (2005) Sea bass (Dicentrarchus labrax) androgen receptor: CDNA cloning, tissue-specific expression, and mRNA levels during early development and sex differentiation. Mol Cell Endocrinol 237:37–48
Brunner B, Hornung U, Shan Z et al (2001) Genomic organization and expression of the doublesex-related gene cluster in vertebrates and detection of putative regulatory regions for DMRT1. Genomics 77:8–17
Chen J, Zhu Z, Hu W (2022) Progress in research on fish sex determining genes. Water Biol Secur 1:100008
CoStat 6.451 (2017) Copyright(c) 1998-2017, CoHort Software. http://www.cohort.com
Dalla Valle L, Lunardi L, Colombo L, Belvedere P (2002) European sea bass (Dicentrarchus labrax L.) cytochrome P450arom: cDNA cloning, expression and genomic organization. J Steroid Biochem Mol Biol 80:25–34
Deloffre LAM, Martins RST, Mylonas CC, Canario AVM (2009) Alternative transcripts of DMRT1 in the European sea bass: expression during gonadal differentiation. Aquaculture 293:89–99
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1
FAO (2019) Fisheries and aquaculture - fisheries and aquaculture - FAO yearbook of fishery and aquaculture statistics. In: FAO. https://www.fao.org/fishery/en/statistics/yearbook. Accessed 21 Mar 2022
Gonzalez-Martinez A, De-Pablos-Heredero C, González M et al (2021) Usefulness of discriminant analysis in the morphometric differentiation of six native freshwater species from Ecuador. Animals. https://doi.org/10.3390/ani11010111
Guan G, Kobayashi T, Nagahama Y (2000) Sexually dimorphic expression of two types of DM (doublesex/Mab-3)-domain genes in a teleost fish, the tilapia (Oreochromis niloticus). Biochem Biophys Res Commun 272:662–666
Guiguen Y, Fostier A, Piferrer F, Chang CF (2010) Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. Gen Comp Endocrinol 165:352–366
Guo Y, Cheng H, Huang X et al (2005) Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem Biophys Res Commun 330:950–957
Halm S, Martínez-Rodríguez G, Rodríguez L et al (2004) Cloning, characterisation, and expression of three oestrogen receptors (ERα, ERβ1 and ERβ2) in the European sea bass, Dicentrarchus labrax. Mol Cell Endocrinol 223:63–75
Halm S, Rocha A, Miura T et al (2007) Anti-Müllerian hormone (AMH/AMH) in the European sea bass: Its gene structure, regulatory elements, and the expression of alternatively-spliced isoforms. Gene 388:148–158
Hofsten J, Olsson PE (2005) Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reprod Biol Endocrinol 3:1–11
Kaleem O, Bio Singou Sabi AF (2021) Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquac Fish 6:535–547
Kaneene JB, Miller RA, Sayan R et al (2007) Considerations when using discriminant function analysis of antimicrobial resistance profiles to identify sources of fecal contamination of surface water in Michigan. Appl Environ Microbiol 73:2878–2890
Kemkes-Grottenthaler A (2005) Sex determination by discriminant analysis: an evaluation of the reliability of patella measurements. Forensic Sci Int 147:129–133
Legendre P, Legendre L (2012) Numerical ecology - P. Elsevier, Legendre, Louis Legendre, Third Engl
Lin A, Xiao S, Xu S et al (2017) Identification of a male-specific DNA marker in the large yellow croaker (Larimichthys crocea). Aquaculture 480:116–122
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Long J, Zheng S, Wang X et al (2020) Role of TGF-β signaling pathway in sex determination and differentiation in fish. J Fish China 44:166–177
Luckenbach JA, Early LW, Rowe AH et al (2005) Aromatase cytochrome P450: cloning, intron variation, and ontogeny of gene expression in southern flounder (Paralichthys lethostigma). J Exp Zool Part A Comp Exp Biol 303A:643–656
Marchand O, Govoroun M, D’Cotta H et al (2000) DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss. Biochim Biophys Acta - Gene Struct Expr 1493:180–187
Martins RST, Deloffre LAM, Mylonas CC et al (2007) Developmental expression of DAX1 in the European sea bass, Dicentrarchus labrax: lack of evidence for sexual dimorphism during sex differentiation. Reprod Biol Endocrinol 5:1–13
Matsuoka MP, van Nes S, Andersen Ø et al (2006) Real-time PCR analysis of ovary- and brain-type aromatase gene expression during Atlantic halibut (Hippoglossus hippoglossus) development. Comp Biochem Physiol Part B Biochem Mol Biol 144:128–135
Panagiotopoulou H, Marzecki K, Gawor J et al (2023) Extensive search of genetic sex markers in Siberian (Acipenser baerii) and Atlantic (A. oxyrinchus) sturgeons. Aquaculture 573:739517
Piferrer F, Blázquez M, Navarro L, González A (2005) Genetic, endocrine, and environmental components of sex determination and differentiation in the European sea bass (Dicentrarchus labrax L.). Gen Comp Endocrinol 142:102–110
Piferrer F, Guiguen Y (2008) Fish gonadogenesis. part II: molecular biology and genomics of sex differentiation. Rev Fish Sci 16:33–53
Saillant E, Chatain B, Menu B et al (2003) Sexual differentiation and juvenile intersexuality in the European sea bass (Dicentrarchus labrax). J Zool 260:53–63
Socorro S, Martins RS, Deloffre L et al (2007) A cDNA for European sea bass (Dicentrachus labrax) 11β-hydroxylase: gene expression during the thermosensitive period and gonadogenesis. Gen Comp Endocrinol 150:164–173
Tudela S, Coll M, Palomera I (2005) Developing an operational reference framework for fisheries management on the basis of a two-dimensional index of ecosystem impact Abstract ICES. Journal of Marine Science 62(3):585–591. https://doi.org/10.1016/j.icesjms.2005.01.008
Vandeputte M, Dupont-Nivet M, Chavanne H, Chatain B (2007) A polygenic hypothesis for sex determination in the European sea bass Dicentrarchus labrax. Genetics 176:1049–1057
Vizziano D, Randuineau G, Baron D et al (2007) Characterization of early molecular sex differentiation in rainbow trout, Oncorhynchus mykiss. Dev Dyn 236:2198–2206
Wootton, R.J. and Smith, C. (2014). Sex determination. In Reproductive Biology of Teleost Fishes (eds R.J. Wootton and C. Smith). https://doi.org/10.1002/9781118891360.CH2
Yamaguchi A, Lee KH, Fujimoto H et al (2006) Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes. Comp Biochem Physiol Part D Genomics Proteomics 1:59–68
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. Samy Y. El-Zaeem put the experimental idea and design and revised the manuscript. Amr El-Hanafy, Alaa A. El-Dahhar, and Sara F. Ghanem revised the manuscript. Ayaat M. Elmaghraby helped and monitored the practical part, writing, and revising the manuscript. Amany M. Hendy performed practical experiments, analyzed the data, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Zaeem, S.Y., El-Hanafy, A., El-Dahhar, A.A. et al. A Novel Investigation for Early Sex Determination in Alive Adult European Seabass (Dicentrarchus labrax) Using cyp19a1a, dmrt1a, and dmrt1b Genes Expression in Tail Fin tissues. Mar Biotechnol (2024). https://doi.org/10.1007/s10126-024-10313-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10126-024-10313-z