Abstract
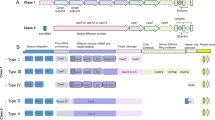
The clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) system has been widely applied in animals as an efficient genome editing tool. However, the technique is difficult to implement in fish cell lines partially due to the lack of efficient promoters to drive the expression of both sgRNA and the Cas9 protein within a single vector. In this study, it was indicated that the zebrafish U6 RNA polymerase III (ZFU6) promoter could efficiently induce tyrosinase (tyr) gene editing and lead to loss of retinal pigments when co-injection with Cas9 mRNA in zebrafish embryo. Furthermore, an optimized all-in-one vector for expression of the CRISPR/Cas9 system in the zebrafish fibroblast cell line (PAC2) was constructed by replacing the human U6 promoter with ZFU6 promoter, basing on the lentiCRISPRV2 system that widely applied in mammal cells. This new vector could successfully target the cellular communication network factor 2a (ctgfa) gene and demonstrated its function in the PAC2 cell. Notably, the vector could also be used to edit the endogenous EMX1 gene in the mammal 293 T cell line, implying its wide application potential. In conclusion, we established a new gene editing tool for zebrafish cell line, which could be a useful in vitro platform for high-throughput analyzing gene function in fish.






Similar content being viewed by others
Data Availability
Data is provided within the manuscript or supplementary information files.
References
Ablain J, Durand EM, Yang S et al (2015) A CRISPR/Cas9 vector system for tissue-specific gene disruption in zebrafish. Dev Cell 32:756–764
Ansai S, Kinoshita M (2014) Targeted mutagenesis using CRISPR/Cas system in medaka. Biol Open 3:362–371
Bogdanove AJ, Voytas DF (2011) TAL effectors: customizable proteins for DNA targeting. Science 333:1843–1846
Chakrapani V, Patra SK, Panda RP et al (2016) Establishing targeted carp TLR22 gene disruption via homologous recombination using CRISPR/Cas9. Dev Comp Immunol 61:242–247
Chang N, Sun C, Gao L et al (2013) Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res 23:465–472
Chanput W, Mes JJ, Wichers HJ (2014) THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 23:37–45
Dehler CE, Boudinot P, Martin SAM et al (2016) Development of an efficient genome editing method by CRISPR/Cas9 in a fish cell line. Mar Biotechnol 18:449–452
DiCarlo JE, Norville JE, Mali P et al (2013) Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res 41:4336–4343
Edvardsen RB, Leininger S, Kleppe L et al (2014) Targeted mutagenesis in Atlantic salmon (Salmo salar L.) using the CRISPR/Cas9 system induces complete knockout individuals in the F0 generation. PLoS One 9:e108622
Elaswad A, Khalil K, Ye Z et al (2018) Effects of CRISPR/Cas9 dosage on TICAM1 and RBL gene mutation rate, embryonic development, hatchability and fry survival in channel catfish. Sci Rep 8:16499
Feng R, Fang L, Cheng Y et al (2015) Retinoic acid homeostasis through aldh1a2 and cyp26a1 mediates meiotic entry in Nile tilapia (Oreochromis niloticus). Sci Rep 5:10131
Gaj T, Gersbach CA, Barbas CF 3rd (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31:397–405
Gratacap RL, Regan T, Dehler CE et al (2020) Efficient CRISPR/Cas9 genome editing in a salmonid fish cell line using a lentivirus delivery system. BMC Biotechnol 20:35
Gratz SJ, Cummings AM, Nguyen JN et al (2013) Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194:1029–1035
Hamar J, Kültz D (2021) An efficient vector-based CRISPR/Cas9 system in an Oreochromis mossambicus cell line using endogenous promoters. Sci Rep 11:7854
Hisano Y, Sakuma T, Nakade S et al (2015) Precise in-frame integration of exogenous DNA mediated by CRISPR/Cas9 system in zebrafish. Sci Rep 5:8841
Hoshijima K, Jurynec MJ, Grunwald DJ (2016) Precise editing of the zebrafish genome made simple and efficient. Dev Cell 36:654–667
Hruscha A, Krawitz P, Rechenberg A et al (2013) Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 140:4982–4987
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157:1262–1278
Hwang WY, Fu Y, Reyon D et al (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227–229
Jao LE, Wente SR, Chen W (2013) Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A 110:13904–13909
Jiang F, Doudna JA (2017) CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys 46:505–529
Jinek M, Chylinski K, Fonfara I et al (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Knapp DJHF, Michaels YS, Jamilly M et al (2019) Decoupling tRNA promoter and processing activities enables specific Pol-II Cas9 guide RNA expression. Nat Commun 10:1490
Li JF, Norville JE, Aach J et al (2013) Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31:688–691
Li M, Yang H, Zhao J et al (2014) Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetics 197:591–599
Liang Z, Chen K, Li T et al (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8:14261
Lin F, Ye X, Lin J et al (2022) Comparative transcriptome analysis between muscle and swim bladder reveals key genes regulating collagen deposition in zebrafish. Aquacult Rep 23:101053
Liu P, Long L, Xiong K et al (2014a) Heritable/conditional genome editing in C. elegans using a CRISPR-Cas9 feeding system. Cell Res 24:886–889
Liu Q, Yuan Y, Zhu F et al (2018) Efficient genome editing using CRISPR/Cas9 ribonucleoprotein approach in cultured Medaka fish cells. Biol Open 7:bio035170
Liu D, Wang ZX, Xiao A et al (2014b) Efficient gene targeting in zebrafish mediated by a zebrafish-codon-optimized cas9 and evaluation of off-targeting effect. J Genet Genomics 41:43–46
Lino CA, Harper JC, Carney JP, Timlin JA (2018) Delivering CRISPR: a review of the challenges and approaches. Drug Deliv 25:1234–1257
Niu Y, Shen B, Cui Y et al (2014) Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156:836–843
Pan Q, Luo J, Jiang Y et al (2022) Efficient gene editing in a medaka (Oryzias latipes) cell line and embryos by SpCas9/tRNA-gRNA. J Zhejiang Univ Sci B 23:74–83
Sakaguchi K, Yoneda M, Sakai N et al (2019) Comprehensive experimental system for a promising model organism candidate for marine teleosts. Sci Rep 9:4948
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32:347–355
Sanjana NE, Shalem O, Zhang F (2014) Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods 11:783–784
Shinya M, Kobayashi K, Masuda A et al (2013) Properties of gene knockdown system by vector-based siRNA in zebrafish. Dev Growth Differ 55:755–765
Su T, Liu F, Gu P et al (2016) A CRISPR-Cas9 assisted non-homologous end-joining strategy for one-step engineering of bacterial genome. Sci Rep 6:37895
Wang H, La Russa M, Qi LS (2016) CRISPR/Cas9 in genome editing and beyond. Annu Rev Biochem 85:227–264
Wang H, Yang H, Shivalila CS et al (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153:910–918
Wargelius A, Leininger S, Skaftnesmo KO et al (2016) Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci Rep 6:21284
Wilding JL, Bodmer WF (2014) Cancer cell lines for drug discovery and development. Cancer Res 74:2377–2384
Xie K, Minkenberg B, Yang Y (2015) Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci U S A 112:3570–3575
Xu L, Zhao L, Gao Y et al (2017) Empower multiplex cell and tissue-specific CRISPR-mediated gene manipulation with self-cleaving ribozymes and tRNA. Nucleic Acids Res 45:e28
Yeh YC, Kinoshita M, Ng TH et al (2017) Using CRISPR/Cas9-mediated gene editing to further explore growth and trade-off effects in myostatin-mutated F4 medaka (Oryzias latipes). Sci Rep 7:11435
Zoppo M, Okoniewski N, Pantelyushin S et al (2021) A ribonucleoprotein transfection strategy for CRISPR/Cas9-mediated gene editing and single cell cloning in rainbow trout cells. Cell Biosci 11:103
Acknowledgements
We are very grateful to Dr. Cuiying Chen from Shantou University for professional advice in this study.
Funding
This work was supported by the National Natural Science Foundation of China (32373108), Natural Science Foundation of Guandong Province (2022A1515010778), Special Fund for Science and Technology of Guangdong Province (STKJ202209036), Department of Education of Guangdong Province (2022ZDZX4006), and Special Fundation for Rural Revitalization of Guangdong Province (Dzxny018).
Author information
Authors and Affiliations
Contributions
Xiaokang Ye: investigation, formal analysis, writing—original draft preparation; Jiali Lin: investigation, data curation; Qiuji Chen: resources; Jiehuan Lv: resources; Chunsheng Liu: resources; Yuping Wang: resources; Shuqi Wang: writing—review and editing; Xiaobo Wen: writing—review and editing; Fan Lin: conception, supervision, writing—review and editing, funding acquirement.
Corresponding author
Ethics declarations
Ethics Approval
All animal procedures were carried out in accordance with the Guideline for the Care and Use of Laboratory Animals of Shantou University.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, X., Lin, J., Chen, Q. et al. An Efficient Vector-Based CRISPR/Cas9 System in Zebrafish Cell Line. Mar Biotechnol (2024). https://doi.org/10.1007/s10126-024-10320-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10126-024-10320-0