Abstract
Widespread environmental pollution caused by the misuse of tetracyclines (TCs) has become a global issue, necessitating the development of water treatment materials for antibiotic removal. Magnetic biochar (MBC) possesses several advantages, including a wide range of raw material sources and low cost, making it a potential adsorbent that overcomes the limitations of biochar (BC) regarding solid–liquid separation. In this study, peanut shell-derived magnetic biochar loaded with Fe3O4 (Fe3O4/BC) was prepared to study its adsorption performance and environmental factors for TCs. The adsorption mechanism was revealed using adsorption isotherms, adsorption kinetics and thermodynamics. The results showed that the total pore volume was increased, and surface oxygen-containing functional groups were formed of that before BC modification. In a wide pH range, Fe3O4/BC showed high adsorption performance for TCs, with an adsorption rate of over 85%. Chemical adsorption was the main adsorption mechanism, including hydrogen bonding, as well as π-π interactions, electrostatic interactions, intrapore diffusion and hydrophobic interactions. Moreover, reusability and obtaining cost of the material were analyzed, demonstrating its promising application prospects. This study will promote the application of Fe3O4/BC in the removal of antibiotics pollutants from water.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Antibiotics are often used as drugs for treatment, disease prevention and aquaculture. Unabsorbed antibiotics enter sewage pipe networks, water bodies and soil environments in the form of prototypes or metabolites. Antibiotics directly contaminate surface water and threaten ecosystems due to the widespread spread of their resistance genes [1–4]. 139 rivers in the United States are polluted by antibiotics with the highest concentration up to 1,900 ng L−1 [5, 6]. In the Yellow River Basin of China, the content of antibiotics ranges between 1.04–67.6 ug/L [7, 8]. Tetracyclines (TCs) were also detected up to several hundreds μg/L in poultry farms, aquaculture ponds, and pharmaceutical wastewater [9]. Even an extremely high concentration of TCs (32.0 mg L−1) occurred in the wastewater of the antibiotic production facility [10]. However, the European Agency for the Evaluation of Medicinal Products (EMEA) has set a threshold value of 10 ng/l for antibiotics in the water environment. Due to the difficult biodegradation and complex molecular structure of antibiotics, it is difficult to completely remove antibiotics from the water environment, and it has become a research hotspot to efficiently remove antibiotics from the water environment [11].
As one of the most widely used and mature techniques, the adsorption method has the advantages of simple operation, low cost, and generally high removal efficiency of pollutants without highly toxic by-products during treatment [12]. Commonly used adsorbents include biochar, zeolite, activated carbon, molecular sieve and metal–organic frameworks [13, 14]. By contrast, biochar, derived from the thermal decomposition of waste biomass, has become one of the most promising adsorbents due to its low cost, wide range of sources, simple preparation, and large surface area [15]. Biochar(BC) has abundant groups, including hydroxyl, carbonyl and carboxyl groups, and its high porosity and large specific surface area facilitate the adsorption of organic pollutants [16]. However, due to the poor solid–liquid separation performance, it is difficult to effectively recycle BC after adsorbing pollutants from water body, posing a risk of secondary pollution of effluent [17]. By magnetizing biochar with magnetic media such as iron and cobalt, the prepared magnetic biochar(MBC) has both excellent adsorption performance and efficient solid–liquid separation performance, which can achieve rapid separation under the action of magnetic field [18], effectively removing pollutants such as oils, polyaromatic hydrocarbons, and organic synthetic dyes [19–21].
TCs are the most widely consumed antimicrobials in the United States and European Union [22]. In order to control adverse environmental effects, researchers have explored the use of MBC to adsorb and remove TCs from water. The main research achievements obtained are summarized in table 1. Current research focuses on material preparation methods and magnetic precursors to improve adsorption properties but pays little attention to the cost of material preparation, which is an important factors affecting the application of such water treatment materials.
Table 1. Main research achievements on MBC adsorption of tetracycline.
| Research orientation | Main research achievements | References |
|---|---|---|
| Preparation methods | • K2FeO4-accelerated hydrothermal carbonization of kelp was studied to manufacture MBC for eliminating TCs and this method also exhibited good generality for corn stalk, coconut shell, banana pseudostem, and pepper stalk as feedstock. | [23] |
| • A facile ball milling technology was proposed to customize the MBC of bagasse loaded with Fe-Al oxide, which has a strong adsorption capacity for TCs. | [24] | |
| • MBC was prepared by pyrolysis of tea waste, KHCO3, and FeCl3·6H2O at different temperatures. The pyrolysis temperature, the more favorable the adsorption of TCs to the prepared material. | [25] | |
| Magnetic precursors and other modified materials | • MBC doped with different iron valences was used for TC adsorption. K2FeO4-doped biochar was the most effective for TCs adsorption, with the best separation performance. | [26] |
| • MBC were produced by one-step pyrolysis/KOH-activation of walnut shell, rice husk and cornstalk with different iron impregnation ratios. MBC prepared with low impregnation ratio (0.3) showed stronger adsorption capacity on tetracycline. | [27] | |
| • Fe/N co-doped MBC were obtained by grinding corncob, CH3COOK, FeCl3·6H2O, and C3H6N6 via one-step synthesis and were applied to remove TCs. CH3COOK had an excellent porous activation ability. | [28] | |
| Research methods and mechanism reveal | • MBC was prepared from poplar sawdust for the removal of TCs. Conductor-like screening model for real solvents (COSMO-RS) reveals that hydrogen bond interaction causes difference adsorption. | [29] |
| • A municipal sludge based Biochar was prepared for removal of TCs. Site energy distribution (SED) results revealed that the surface of BC was more homogeneous while the surface of MBC was more heterogeneous at higher temperature. | [30] | |
| Obtaining cost | • MBC was prepared by the coupling hydrothermal carbonization and pyrolysis activation of starch-rich rice waste using ZnCl2 and FeCl3. The preparation cost and the material consumption per ton of wastewater treatment were estimated. | [31] |
Peanuts play a significant role as an oil crop. The global peanut production in 2019 was 48.8 million tons, and the peanut shells production reached 15 million tons [32]. Unfortunately, except for a small portion used as feed or chemical raw materials, the majority of peanut shells are directly burned or discarded in farmland, not only polluting the environment, but also wasting the resources. Peanut shells are known to consist of microfibers rich in cellulose and hemicellulose, with low ash content [33]. In recent years, peanut shells have been used as substrates for the synthesis of various kinds of adsorbents for wastewater treatment [34–37], but there are few reports on the adsorption of TCs.
Specific to TCs in the detected antibiotics from water body, this study focused on the preparation of Fe3O4/BC using peanut shells biochar as a substrate. The influencing factors on organic pollutant adsorption were investigated, the adsorption mechanism was explored, and the production cost was estimated. The primary objectives of this study are to recycle agricultural waste of peanut shells, effectively control the ecological and environmental risks of TCs, provide a theoretical basis for the application of MBC in the removal of antibiotics in water bodies, and contribute to the sustainable development of resources and the environment.
2. Materials and methods
2.1. Preparation of materials
The co-precipitation method was employed to prepare magnetic biochar materials. After being cleaned, dried, crushed and screened through a 200-mesh screen, peanut shells were bagged. The powdered BC was obtained through pyrolysis at 500 °C. After adding 0.05g polyethylene glycol to 1g BC, stirring in water for 20 min and still standing for 30 min, 2g FeCl3 and FeSO4 were added in a 2:1 ratio, stirring while adding ammonia dropwise. The resulting mixture was then heated and stirred at 55 °C for 30 min. The product was separated by magnet, washed with absolute ethanol, and dried at 80 °C for 4h to obtain magnetic biochar [38].
2.2. Sample characterization
Scanning electron microscopy (SEM) was used to characterize the micromorphology of Fe3O4/BC. X-ray diffraction detector (XRD) was used to analyze the crystal structure of the material. X-ray photoelectron spectroscopy (XPS) was used to analyze the changes in the chemical composition, chemical valence and surface element content of the magnetic biochar sample. The energy standard C 1s = 284.80eV was used for charge correction, and then peak-differentiating and imitating were performed through the element spectrum to obtain the relative content of the same element in different valence states. Fourier transform infrared (FT-IR) spectrum (IS5) was used to determine the chemical structure in the zone of 4,000–400 cm−1. Micromeritics ASAP2020 HD 88 specific surface area analyzer was used to analyze the BET parameter of Fe3O4/BC [39]. The detailed characterization results of Fe3O4/BC had been published in another article [40] of our research group.
2.3. Tetracycline adsorption experiment
To simulate real wastewater conditions, the effects of adsorbent dosage of 5 ∼ 25 mg L−1, pH−1 value of 3 ∼ 11, initial concentration of 10 ∼ 60 mg and temperature of 5 ∼ 35 °C on the adsorption capacity were investigated, and the adsorption capacity of Fe3O4/BC for TC was evaluated accordingly. To ensure the accuracy of the experimental results, parallel experiments were carried out to investigate the influence of environmental factors on the adsorption properties. Each experiment was repeated for 3 times. All batch experiments were performed in 50 ml conical bottles. The conical bottles were placed in a magnetic stirrer with a rotation speed of 150 rpm for adsorption experiments. After the reaction, the mixture was separated by magnet, and the effect of Fe3O4/BC dosage on the performance of the TC solution was obtained [41].The filtrate was filtered through a 0.45 μm membrane. The maximum absorption wavelength of TC was 356nm, and the absorbance of filtrate was measured by UV-visible spectrophotometer.
2.4. Analysis of the removal mechanism of tetracycline by magnetic biochar
After Fe3O4/BC adsorbed TC in water, the isotherm model, adsorption kinetic model and thermodynamics were respectively used to analyze the adsorption process of TC by Fe3O4/BC. X-ray photoelectron spectroscopy (XPS) was used to analyze the changes in chemical composition, chemical valence and surface element content of magnetic biochar samples before and after adsorption and removal of pollutants. Fourier transform infrared (FT-IR) spectroscopy (IS5) was used to determine the chemical structure in the zones of 4,000–400 cm−1. The adsorption mechanism of TC by Fe3O4/BC was explored [42].
3. Results and discussion
3.1. Effect of Fe3O4/BC on adsorption performance of tetracycline
The dosage of Fe3O4/BC increased from 5mg to 25mg/L (figure 1), and the adsorption rate of TC gradually increased. When the dosage of Fe3O4/BC reached the critical level, the removal rate of TC showed no significant changes. This can be attributed to the fact that increasing the amount of material provides more active sites for the removal of TC. Once the adsorbent dosage exceeded 20mg, the additional amount of Fe3O4/BC had no significant effect on the removal of TC. Therefore, the optimal of Fe3O4/BC was 20 mg.
Figure 1. Effect of Fe3O4/BC on TC removal.
Download figure:
Standard image High-resolution image3.2. Effect of pH on adsorption performance of tetracycline
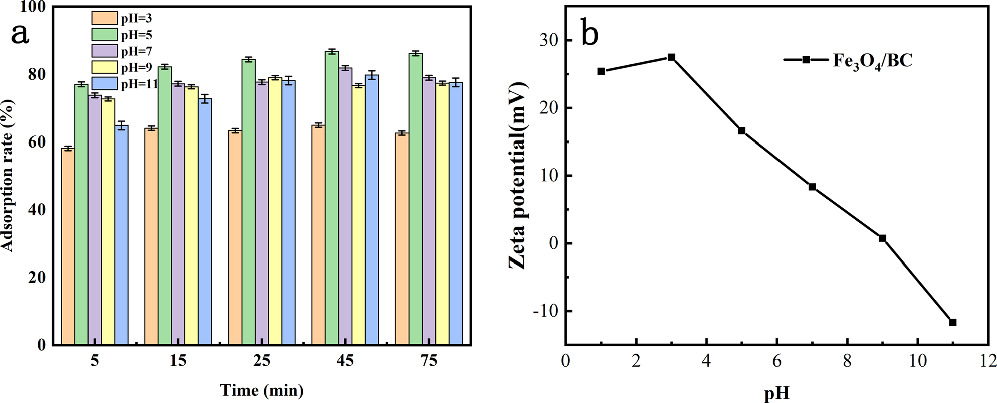
Changes in the pH value of the solution can alter the functional groups of Fe3O4/BC and TC, and the changes in pH value mainly affected the charge property of Fe3O4/BC, leading to variations in the degree of protonation of the adsorbent. Tetracycline has three hydrolysis constants (pKa) at different stages, namely pKa1 = 3.3, pKa2 = 7.7, and pKa3 = 9.7. With pH less than 3.3, TC is cationic; within the pH range of 3.3–7.7, TC is zwitterionic; and with pH above 7.7, TC is anionic [43, 44]. When the pH value of the solution in the experiment was 5, the adsorption capacity and removal rate of Fe3O4/BC in the adsorption process reached a maximum of 85% (figure 2(a)). This was because at pH 5, Fe3O4/BC was positively charged, TC mainly existed in the form of zwitterions, and the electrostatic attraction was conducive to the adsorption of TC on Fe3O4/BC. As the pH gradually increased, the negative charge of Fe3O4/BC gradually accumulated, and TC was dominated by the negative charge [45]. Therefore, there was electrostatic repulsion between Fe3O4/BC and TC, and as the pH value continued to increase, the repulsion would be greater, resulting in a decrease in the removal rate [46]. To maximize the removal of TC by Fe3O4/BC, the pH value was set to 5 in the subsequent adsorption experiment.
Figure 2. Effect of solution pH on TC adsorption (a), Zeta potential pattern (b).
Download figure:
Standard image High-resolution imageThe hydrochloride form of tetracycline is relatively stable, and its production wastewater is the main source of environmental pollutants, and the water quality is usually weakly acidic [47, 48]. Song et al [49] used transition metal activation PMS method to discuss the actual treatment effect and removal mechanism of pharmaceutical wastewater with pH = 4. In order to further explore the practical application effect of the prepared material, this study carried out a discussion on the influencing factors of subsequent removal effect under weak acidic conditions (pH = 5). In addition to the strong acidic environment (pH = 3), the removal rate of tetracycline after 30 min of use of the material in this study was more than 75%, and it also showed a broad acid–base application range (pH 5 ∼ 11), which has a certain application prospect.
3.3. Effect of initial concentration on adsorption performance of tetracycline
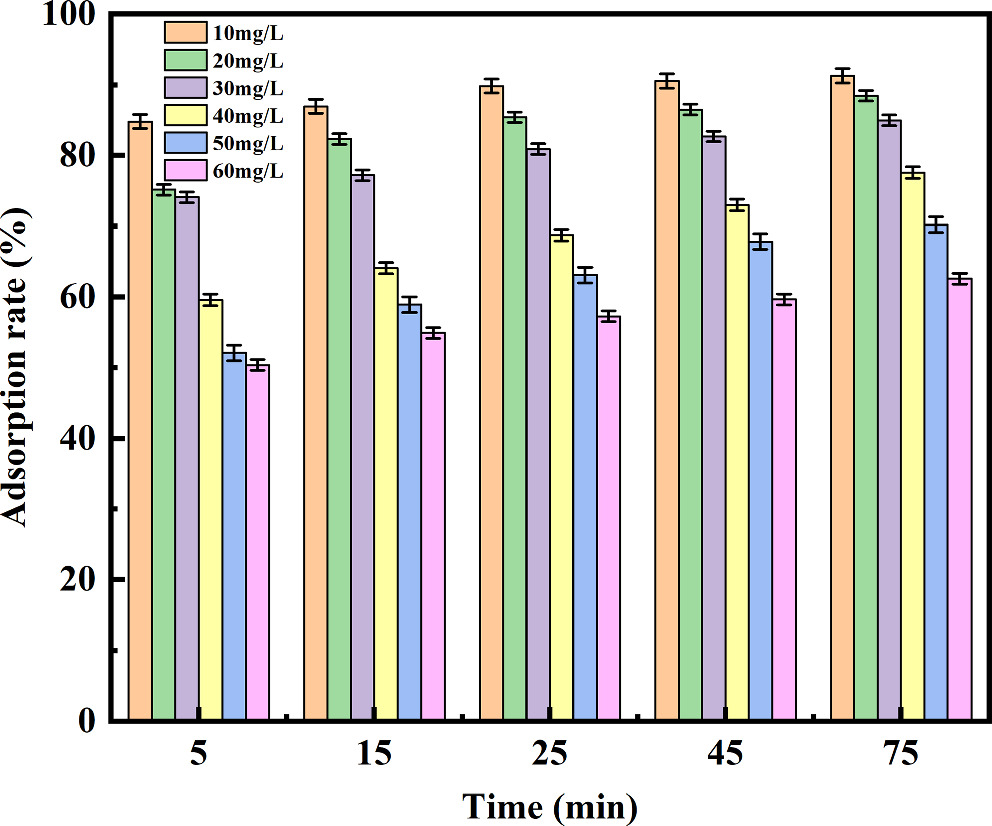
When the concentration of TC solution increased from 10 mg L−1 to 60 mg/l under the fixed adsorbent dosage, the removal rate of TC decreased from 89% to 51%,(figure 3). This showed that the adsorption of Fe3O4/BC was more conducive to the treatment of low concentration TC wastewater. In addition, under the same concentration of adsorbent, the adsorption capacity of Fe3O4/BC also continued to increase with the increasing of the initial concentration of pollutants. However, the adsorption rate showed a slightly downward trend. This was because the increased concentration of TC substrate made TC and Fe3O4/BC fully mixed, increasing the adsorption area and improving the adsorption efficiency. Nevertheless, excessive TC concentration increased the load capacity of the adsorption site in Fe3O4/BC, which would lead to a slight decrease in the adsorption rate [50, 51]. In order to maintained a high removal rate and obtained a stable adsorption capacity, the initial concentration of TC was controlled at 20 m g /L.
Figure 3. Effect of different initial concentrations on TC adsorption.
Download figure:
Standard image High-resolution image3.4. Effect of temperature on adsorption performance of tetracycline by magnetic biochar
The removal rates at 5 °C and 35 °C were 83% and 89%, respectively, representing an increase of approximately 6% (figure 4). The results of this experiment showed that the increased temperature was conducive to the adsorption of TC by Fe3O4/BC, and the high temperature was conducive to the diffusion of organic pollutant molecules in the pores of biochar and the contact of surface sites, which ultimately leaded to an increasing in adsorption capacity [52, 53]. However, considering energy saving, an ambient temperature of 25 °C was selected instead of maintaining the high temperature used in this experiment, which required heating the wastewater.
Figure 4. Effect of temperature on Fe3O4/BC adsorption of TC.
Download figure:
Standard image High-resolution image3.5. Analysis of the removal mechanism of tetracycline by magnetic biochar
As an adsorbent, Fe3O4/BC could well adsorb TC in water. Therefore, the isotherm model, adsorption kinetic model and thermodynamics were used to analyze the adsorption mechanism of TC by Fe3O4/BC.
3.5.1. Kinetic analysis of tetracycline by magnetic biochar.
The results of quasi-primary and quasi-secondary kinetic model fitting were shown in table 2. The quasi-first-order kinetic equation showed the process of physical adsorption, while the quasi-second-order kinetic model showed chemical adsorption and explained the processes of outer membrane diffusion in the liquid as well as surface adsorption and intraparticle diffusion [54, 55]. As can be seen from table 2, when tetracycline concentrations were 10 mg L−1, 20 mg/l and 30 mg L−1 respectively, the adsorption of TC by Fe3O4/BC was better fitted with the quasi-second-order kinetic equation than the quasi-first-order kinetic equation, wiht R2 values reaching 0.986, 0.998 and 0.999, respectively. It can be inferred that the adsorption of TC by Fe3O4/BC is mainly chemical adsorption.
Table 2. Kinetic parameters.
| Quasi-primary-order kinetic parameters | Quasi-secondary -order kinetic parameters | ||||||
|---|---|---|---|---|---|---|---|
| Qe | K1 | R2 | Qe | K1 | R2 | ||
| 30 mg L−1 | 33.539 | 0.015 | 0.929 | 30 mg L−1 | 34 | 0.030 | 0.999 |
| 20 mg L−1 | 22.118 | 0.009 | 0.653 | 20 mg L−1 | 23 | 0.044 | 0.998 |
| 10 mg L−1 | 10.112 | 2.865 | 0.071 | 10 mg L−1 | 11 | 0.095 | 0.986 |
The intraparticle diffusion model reflected the adsorption mechanism and velocity control steps during the adsorption of TC by Fe3O4/BC (figure 5(b)). In this experiment, there were two stages of adsorption of TC by Fe3O4/BC. Overall, the slope of the adsorption stage could be seen as k1 > k2, indicating that the diffusion rate of TC on the Fe3O4/BC surface gradually decreased until reaching equilibrium [56]. The fitting degree of adsorbent Fe3O4/BC in stage 2 was low, because the adsorption of TC pollutants on the surface of Fe3O4/BC at this stage was more complicated, and there were many influencing factors. For stage 1, the possible reason was that the environment in the pores of the material was relatively stable, facilitating smooth diffusion of the liquid film towards the surface of the adsorbent [57, 58].
Figure 5. Quasi-secondary kinetic model (a), Intraparticle diffusion model (b).
Download figure:
Standard image High-resolution image3.5.2. Isotherm analysis of adsorption of tetracycline by magnetic biochar
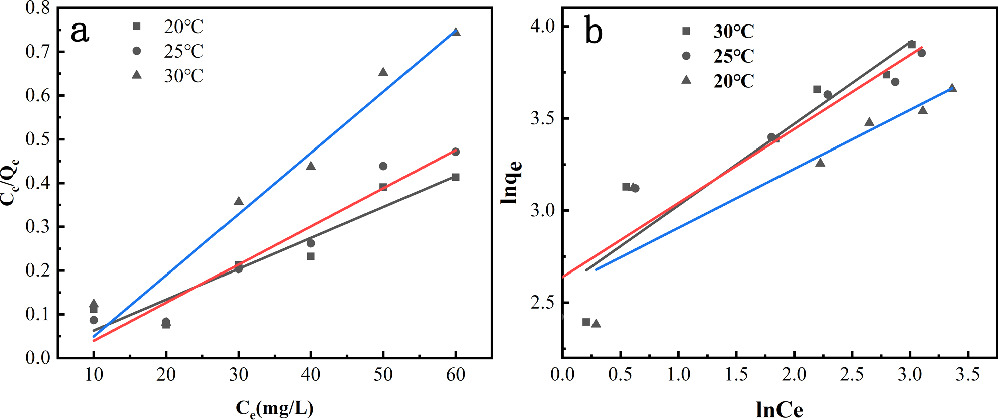
Adsorption isotherm model fitting was employed to analyze the relationship between TC concentration and adsorption capacity, moreover, discuss the theoretical maximum adsorption capacity of TC by Fe3O4/BC. The adsorption isotherm model described the distribution of TC molecules in the solid phase and liquid phase when the adsorption reaction reached equilibrium (figure 6). The results of the isotherm fitting equation for Fe3O4/BC adsorption of TC were shown in table 3.
Figure 6. Adsorption isotherm Langmuir model (a), Adsorption isotherm Frcundlich model (b).
Download figure:
Standard image High-resolution imageTable 3. Parameters of the adsorption isotherm model.
| Langmuir isothermal equation | Freundlich isothermal equation | ||||||
|---|---|---|---|---|---|---|---|
| Qm/(mg/kg) | KL | R2 | n | KF | R2 | ||
| 30 °C | 142.86 | 0.224 | 0.962 | 30 °C | 2.257 | 13.263 | 0.845 |
| 25 °C | 125 | 0.271 | 0.980 | 25 °C | 2.494 | 13.999 | 0.898 |
| 20 °C | 71.43 | 0.276 | 0.976 | 20 °C | 3.125 | 13.277 | 0.755 |
As can be seen from the table, the R2 values obtained from fitting the Langmuir isothermal equation range from 0.962 to 0.980, which is higher the Freundlich equation. Therefore, Langmuir model is more suitable to describe the adsorption process of TC by Fe3O4/BC. The isothermal equation described by Langmuir is a monomolecular adsorption equation. The homogeneous monolayer forms when TC was adsorbed at a fixed number of sites on the Fe3O4/BC surface [59, 60]. Therefore, the adsorption of TC by Fe3O4/BC was mainly monolayer adsorption on the surface of the material. Zhang et al [31] investigated the adsorption process of TC by magnetic biochar in sludge. The fitted R2 (0.8513 ∼ 0.9304) of the Friedrich equation was higher than that of the Friedrich equation (R2 0.7737 ∼ 0.9088), suggesting that the adsorption of TC by materials was suitable to be described by the Friedrich equation. The process is mainly a homogeneous chemisorption process on a single layer surface. In addition, Xiang's research results also that the adsorption of lignin impregnated wheat straw biochar to TC followed the Langmuir model as a homogeneous monolayer adsorption process [61].
3.5.3. Thermodynamic analysis of adsorption of tetracycline by magnetic biochar
The temperature has a certain effect on the adsorption process, so it is necessary to further understand the effect of temperature on the adsorption of TC by Fe3O4/BC [62]. Thermodynamic analysis was used to analyze the adsorption process. According to table 4, Gibbs free energy △G < 0 indicated that the adsorption of TC in water Fe3O4/BC was a spontaneous process. The enthalpy change △H > 0 indicated that the process of adsorption of TC by Fe3O4/BC was an endothermic reaction. The entropy value △S > 0 indicated that the adsorption of TC by Fe3O4/BC was a process of disorder and randomization [63].
Table 4. Thermodynamic parameters of Fe3O4/BC adsorption of TC.
| Concentration | Tempreture/k | Adsorption potential /(kJ/mol) | △G/(kJ/mol) | △H/(kJ/mol) | △S/[J(mol·K)−1] |
|---|---|---|---|---|---|
| 293 | 0.06 | −0.055 | 0.8 | 0.005 | |
| 20 mg L−1 | 298 | 0.06 | −0.056 | ||
| 303 | 0.06 | −0.057 |
3.6. Analysis of the adsorption mechanism of tetracycline by magnetic biochar
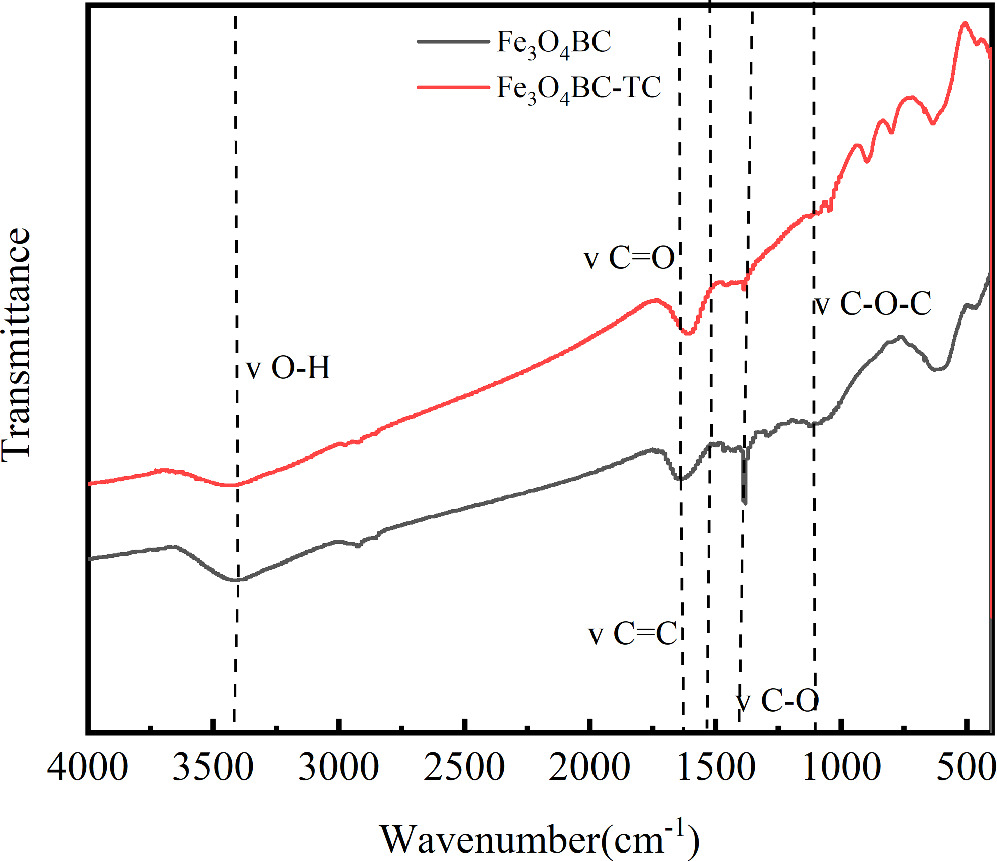
Fe3O4/BC had characteristic absorption peaks of benzene ring both before and after adsorption as in the figure 7 (approximately 3436 cm−1 before adsorption and approximately 3460 cm−1 after adsorption). Absorption peaks caused by C–O single-bond telescopic vibration connected by phenolic hydroxyl group and aromatic ring (1380 cm−1 before adsorption and 1390 cm−1 after adsorption), and absorption peaks caused by O–H bond waving in the benzene ring (520 cm−1 before adsorption and 517 cm−1 after adsorption). After adsorption, the functional groups shifted, indicating that these functional groups participated in the adsorption reaction process as π electron acceptors [64]. In addition, iron played a flocculation role in the carbonization process, leading to an obvious aromatic structure of the biochar framework, and the aromatic structure in the carbonized biomass tended to have π-π interaction with the aromatic ring structure in TC [65]. Hydrogen bonding was established between the phenolic group of TC and the oxygen-containing carbon group to promote the adsorption process, and after Fe3O4/BC adsorbed TC, the functional group composition showed little change, although the absorption peak weakened [66].
Figure 7. FT-IR diagram after adsorption of MG by Fe3O4/BC.
Download figure:
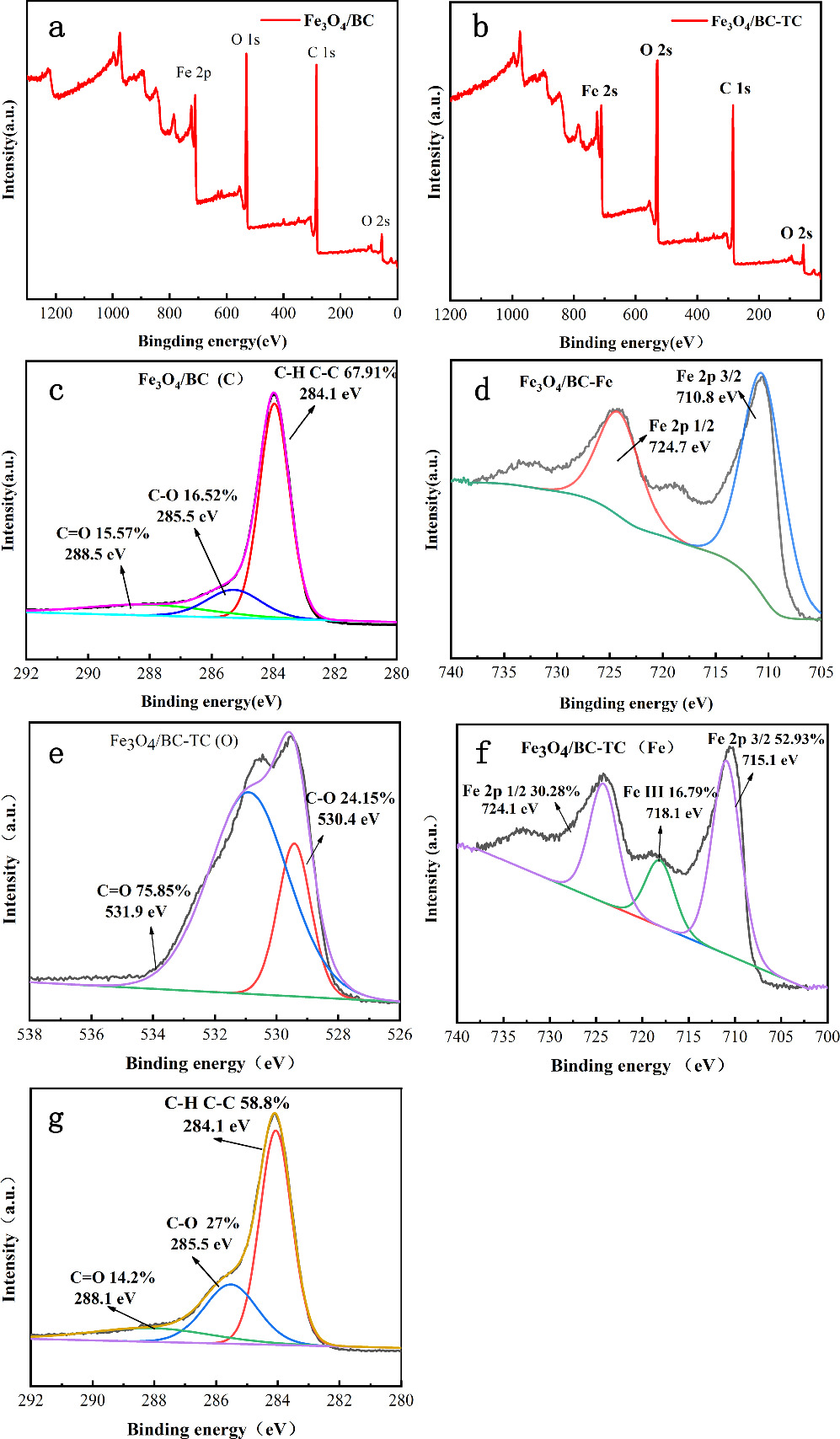
Standard image High-resolution imageThe XPS diagram after Fe3O4/BC adsorbs TC was shown in figure 8. According to the peak fitting, after the adsorption of TC by Fe3O4/BC, C could be decomposed into 3 peaks: 288.1 eV, 285.5 eV and 284.1 eV, corresponding to functional group such as C–C/C–H, C–O and C=O, respectively. O could be decomposed into two peaks: 531.9 eV and 530.4 eV, corresponding to functional groups such as C–O and C=O. After the adsorption of TC by Fe3O4/BC, the position and relative proportion of C and O binding energy of Fe3O4/BC changed, particularly in the oxygen-containing functional group. The Fe characteristic peaks corresponded to Fe 2p 3/2 and Fe 2p 1/2, respectively. The peak separation of 13.8 eV in this spectrum was similar to that of standard Fe2O3. The additional satellite peak at 718.1 eV further demonstrated the presence of Fe3+ in the sample [67]. According to the above analysis, there were functional groups such as hydroxyl, carboxyl and phenolic groups in magnetic biochar [68]. These changes involved aromatic framework vibration, C–H tensile vibration, and C–O tensile vibration. The mechanism showed that the adsorption process of TC by Fe3O4/BC involved hydrogen bonding and there were π-π interactions, electrostatic interactions, pore diffusion and hydrophobic interactions [69, 70].
Figure 8. XPS diagram: Fe3O4/BC(a), Fe3O4/BC adsorption TC(b), Fe3O4/BC C, Fe element (c), (d) Fe3O4/BC adsorption of TC after C, Fe, O element (e), (f), (g).
Download figure:
Standard image High-resolution image3.7. Reusability analysis
The reusability performance of Fe3O4/BC was shown in figure 9. With the increasing in adsorption–desorption cycles, the adsorption efficiency of tetracycline by Fe3O4/BC gradually decreased, and the adsorption efficiency still maintained between 74%–88% after four adsorption–desorption cycles. In addition, we measured the weight of the material after four cycles, and found that it still maintained more than 89% of the initial weight, indicating a good recovery efficiency. The reusability is an important factor in determining the economic and feasibility of adsorbent materials in practical engineering applications [71]. Obviously, Fe3O4/BC demonstrated excellent reusablility for adsorption, making it a highly efficient adsorbent for TC removel in water treatment [72].
Figure 9. Number of reusable times of Fe3O4/BC.
Download figure:
Standard image High-resolution image3.8. Obtaining costs estimation
The economic cost is an important factor affecting the wide application of magnetic biochar. Therefore, it is necessary to estimate the obtaining costs. Factors such as the cost of the precursor, the activating agent, and the cost of energy contribute to the overall net cost of magnetic biochar preparation [73]. The feasibility of extracting biochar from peanut shell was evaluated by a simple cost analysis method. Table 5 shows the cost of preparing 1 kg of magnetic biochar. This gross cost is in the range of those previously reported for other biochar adsorbents which typically range from $0.56 to $5.91 per kg of adsorbent [31, 74–76]. The energy expenditure consumed by the use of electricity for pyrolysis, drying, stirring and other processes is the most important cost, accounting for over 55%. In order to expand the scope of application in the future, it is crucial to reduce energy consumption and further minimizing the preparation cost.
Table 5. Obtaining costs of the material.
| Item | Price(USD)/kg |
|---|---|
| FeCl3 | 0.8 |
| FeSO4 | 0.5 |
| Ammonia | 0.1 |
| Absolute ethanol | 0.2 |
| De-ionized water | 0.1 |
| Energy consumption(including pyrolysis, drying, grinding, stirring) | 2.9 |
| Transportation | 0.6 |
| Gross cost | 5.2 |
4. Conclusion
With the development of industry and human activities, water pollution caused by antibiotics has become increasingly severe. In this study, powdered biochar (BC) was successfully prepared using peanut shells as the main substrate through high-temperature pyrolysis at 500 °C. Subsequently, Fe3O4 particles were uniformly loaded onto BC, resulting in the successful preparation of Fe3O4/BC.
The adsorption of antibiotics by Fe3O4/BC displayed adaptability over a wide range of pH (3–11). Under the conditions of initial concentration of TC of 20 mg L−1, Fe3O4/BC dosage of 20 mg, pH of 5, and temperature of 25 °C, the removal rate of antibiotics TC exceeded 85%. Chemical adsorption was the main adsorption mechanism, including hydrogen bonding, along with π-π interactions, electrostatic interactions, intrapore diffusion and hydrophobic interactions.
Fe3O4/BC exhibited excellent performance in removing TC from water, demonstrating effective solid–liquid separation capabilities. The removal rate remained above 74% after 4 consecutive uses. Cost estimates and analysis highlighted energy consumption as the most significant factor, emphasizing the need for future improvements in the preparation methods to reduce energy consumption. This study provides valuable insights for enhancing the removal effeciency of antibiotic pollutants in water.
Acknowledgments
This work was supported by Henan Province Science and Technology Attack Plan Project (232102320105, 242102320075, 242102321069, 242102321076). This study was also supported by Henan Engineering Technology Research Center of Eco-Environmental Damage Identification and Restoration, and Zhengzhou Key Laboratory of Watershed Environmental Treatment and Zhengzhou Key Laboratory of Environmental Functional Materials.
Data availability statement
The data that support the findings of this study are openly available at the following URL/DOI: https://doi.org/10.1088/2053-1591/acf756.
Conflicts of interest
The authors declare that they have no conflicts of interest.