Abstract
Background
Acidic environments naturally occur worldwide and uncontrolled use of agricultural practices may also cause acidification of soils. The development of acidic conditions disturbs the establishment of efficient microbial populations in their natural niches. The survival of Enterobacter species under acidic stress remains poorly understood.
Objective
This study aimed to investigate the survival of an environmental isolate Enterobacter sp. S-33 under acidic stress and to identify the various genes involved in stress protection at the global gene transcription level. The obtained results provide new targets that will allow understanding the in-depth mechanisms involved in the adaptation of bacteria to environmental pH changes.
Methods
We used the next-generation sequencing (NGS) method to analyze the expression (up-regulation & down-regulation) of genes under varying pH conditions.
Results
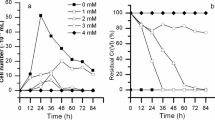
A total of 4214 genes were differentially expressed under acidic conditions (pH 5.0), with 294 up-regulated and 167 down-regulated. At pH 6.0, 50 genes were significantly expressed, of which 34 and 16 were identified as up-regulated and down-regulated, respectively. Many of the up-regulated genes were involved in carbohydrate metabolism, amino acid transport & metabolism, and the most down-regulated genes were related to post-translational modification, lipid transport & metabolism, etc. The observed transcriptomic regulation of genes and pathways identified that Enterobacter reduced its post-translational modification, lipid transport & metabolism, and increased carbohydrate metabolism, amino acid metabolism & transport, energy production & conversion to adapt and grow in acidic stress.
Conclusions
The present work provides in-depth information on the characterization of genes associated with tolerance or adaptation to acidic stress of Enterobacter bacterium.








Similar content being viewed by others
References
Akbari M, Bakhshi B, NajarPeerayeh S (2016) Particular distribution of Enterobacter cloacae strains isolated from urinary tract infection within clonal complexes. Iran Biomed J 20:49–55
Allen LAH (1999) Intracellular niches for extracellular bacteria: lessons from Helicobacter pylori. J Leukoc Biol 66:753–756
Barret M, Egan F, Fargier E, Morrissey JP, O’Gara F (2011) Genomic analysis of the type VI secretion systems in Pseudomonas spp.: novel clusters and putative effectors uncovered. Microbiol 157:1726–1739
Boles BR, Singh PK (2008) Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc Natl Acad SciUSA 105:12503–12508
Bordeleau L, Prevost D (1994) Nodulation and nitrogen fixation in extreme environments. Plant Soil 161:115–125
Brígido C, Oliveira S (2013) Most acid-tolerant chickpea mesorhizobia show induction of major chaperone genes upon acid shock. Microb Ecol 65:145–153
Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW (1999) Control of acid resistance in Escherichia coli. J Bacteriol 181(11):3525–3535
Chen DD, Ahmad M, Liu Y, Wang S, Liu BB, Guo SX (2021) Transcriptomic responses of haloalkalitolerant bacterium Egicoccus halophilus EGI 80432T to highly alkaline stress. Extremophiles 25:459–470
Cowan SW, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit RA, Jansonius JN, Rosenbusch JP (1992) Crystal structures explain functional properties of two E. coli porins. Nature 358:727–733
Dancer GI, Mah JH, Rhee MS, Hwang IG, Kang DH (2009) Resistance of Enterobacter sakazakii (Cronobacter spp.) to environmental stresses. J Appl Microbiol 107:1606–1614
Das P, Behera BK, Chatterjee S, Das BK, Mohapatra T (2020) De novo transcriptome analysis of halotolerant bacterium Staphylococcus sp. strain P-TSB-70 isolated from East coast of India: In search of salt stress tolerant genes. PLoS One 15:e0228199. https://doi.org/10.1371/journal.pone.0228199
de Lucena DK, Pühler A, Weidner S (2010) The role of sigma factor RpoH1 in the pH stress response of Sinorhizobium meliloti. BMC Microbiol 10:1–17
Deibel RH (1964) Utilization of arginine as an energy source for the growth of Streptococcus faecalis. J Bacteriol 87:988–992
Deutscher J, Francke C, Postma PW (2006) How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031
Draghi WO, Del Papa MF, Hellweg C, Watt SA, Watt TF, Barsch A, Lozano MJ, Lagares A Jr, Salas ME et al (2016) A consolidated analysis of the physiologic and molecular responses induced under acid stress in the legume-symbiont model-soil bacterium Sinorhizobium meliloti. Sci Rep 6(1):1–18
Flamez C, Ricard I, Arafah S, Simonet M, Marceau M (2008) Phenotypic analysis of Yersinia pseudotuberculosis 32777 response regulator mutants: New insights into two-component system regulon plasticity in bacteria. Int J Med Microbiol 298:193–207
Frees D, Chastanet A, Qazi S, Sørensen K, Hill P, Msadek T, Ingmer H (2004) Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol Microbiol 54:1445–1462
Garavito RM, Rosenbusch JP (1980) Three-dimensional crystals of an integral membrane protein: an initial x-ray analysis. J Cell Biol 86:327–329
Gerken HG (2009) Novel aspects of bacterial envelope response pathways. PhD dissertation, publication 3391808. Arizona State University, Tempe, AZ
Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94:73–82
Graham PH (1992) Stress tolerance in Rhizobium and Bradyrhizobium, and nodulation under adverse soil conditions. Can J Microbiol 38:475–484
Gray LR, Tompkins SC, Taylor EB (2014) Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 71:2577–2604
Guo J, Ma ZP, Gao JS, Zhao JH, Wei L, Liu J, Xu N (2019) Recent advances of pH homeostasis mechanisms in Corynebacterium glutamicum. World J Microbiol Biotechnol 35:192
Hagting A, Kunji ER, Leenhouts KJ, Poolman B, Konings WN (1994) The di-and tripeptide transport protein of Lactococcus lactis. A new type of bacterial peptide transporter. Jbiol Chem 269:11391–11399
Hellweg C, Pühler A, Weidner S (2009) The time course of the transcriptomic response of Sinorhizobium meliloti 1021 following a shift to acidic pH. BMC Microbiol 9(1):1–16
Hickey EW, Hirshfield IN (1990) Low-pH-induced effects on patterns of protein synthesis and on internal pH in Escherichia coli and Salmonella typhimurium. Appl Environ Microbiol 56:1038–1045
Jugder BE, Batista JH, Gibson JA, Cunningham PM, Asara JM, Watnick PI (2022) Vibrio cholerae high cell density quorum sensing activates the host intestinal innate immune response. Cell Rep 40:111368
Koedooder C, Gueneugues A, Van Geersdaële R, Vergé V, Bouget FY, Labreuche Y, Obernosterer I, Blain S (2018) The role of the glyoxylate shunt in the acclimation to iron limitation in marine heterotrophic bacteria. Front in Mar Sci 5:435
Kumari K, Aggarwal Y, Singh RP (2023) Molecular characterization and in-depth genomic analysis to unravel the pathogenic features of an environmental isolate Enterobacter sp. S-33. Int Microbiol https://doi.org/10.1007/s10123-023-00461-y
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359
Laranjo M, Alexandre A, Oliveira S (2014) Genes commonly involved in acid tolerance are not overexpressed in the plant microsymbiont Mesorhizobium loti MAFF303099 upon acidic shock. Appl Microbiol Biotechnol 98:7137–7147
Lemos JA, Burne RA (2008) A model of efficiency: stress tolerance by Streptococcus mutans. Microbiol 154:3247–3255
Liaw J, Hong G, Davies C, Elmi A, Sima F, Stratakos A, Stef L, Pet I, Hachani A (2019) The Campylobacter jejuni type VI secretion system enhances the oxidative stress response and host colonization. Front Microbiol 10:2864
Lourdault K, Cerqueira GM, Wunder EA Jr, Picardeau M (2011) Inactivation of clpB in the pathogen Leptospira interrogans reduces virulence and resistance to stress conditions. Infect Immun 79:3711–3717
Love M, Anders S, Huber W (2014) Differential analysis of count data–the DESeq2 package. Genome Biol 15:10–1186
Mamo G (2020) Challenges and adaptations of life in alkaline habitats. Adv Biochem Eng Biotechnol 172:85–134
Manson MD, Blank V, Brade G, Higgins CF (1986) Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature 321:253–256
Martikainen P, De Boer W (1993) Nitrous oxide production and nitrification in acidic soil from a Dutch coniferous forest. Soil Biol Biochem 25:343–347
Matsui R, Cvitkovitch D (2010) Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol 5:403–417
Metselaar KI, den Besten HM, Boekhorst J, van Hijum SA, Zwietering MH, Abee T (2015) Diversity of acid stress resistant variants of Listeria monocytogenes and the potential role of ribosomal protein S21 encoded by rpsU. FrontMicrobiol 6:422
Mitsui H, Sato T, Sato Y, Ito N, Minamisawa K (2004) Sinorhizobium meliloti RpoH1 is required for effective nitrogen-fixing symbiosis with alfalfa. Mol Genet Genom 271:416–425
Msimbira LA, Smith DL (2020) The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front Sustain Food Syst 4:106
Nag S, Das S, Chaudhuri K (2005) In vivo induced clpB1 gene of Vibrio cholerae is involved in different stress responses and affects in vivo cholera toxin production. BiochemBiophys Res Commun 331:1365–1373
Nambi S, Long JE, Mishra BB, Baker R, Murphy KC, Olive AJ, Nguyen HP, Shaffer SA, Sassetti CM (2015) The oxidative stress network of Mycobacterium tuberculosis reveals coordination between radical detoxification systems. Cell Host Microbe 17:829–837
Narita SI (2011) ABC transporters involved in the biogenesis of the outer membrane in gram-negative bacteria. Biosci Biotechnol Biochem 75:1044–1054
Nilsson JF, Castellani LG, Draghi WO, Mogro EG, Wibberg D, Winkler A, Hansen LH, Schlüter A, Pühler A, Kalinowski J et al (2021) Global transcriptome analysis of Rhizobium favelukesii LPU83 in response to acid stress. FEMS Microbiol Ecol. 97:fiaa235
Orr HA (2009) Fitness and its role in evolutionary genetics. Nat Rev Genet 10:531–539
Paauw A, Caspers MP, Leverstein-van Hall MA, Schuren FH, Montijn RC, Verhoef J, Fluit AC (2009) Identification of resistance and virulence factors in an epidemic Enterobacter hormaechei outbreak strain. Microbiol 155:1478–1488
Padan E, Tzubery T, Herz K, Kozachkov L, Rimon A, Galili L (2004) NhaA of Escherichia coli, as a model of a pH-regulated Na+/H+ antiporter. Biochim et Biophys Acta (BBA)-Bioenerge 1658:2–13
Padan E, Bibi E, Ito M, Krulwich TA (2005) Alkaline pH homeostasis in bacteria: new insights. Biochim Biophys Acta - Biomembr 1717:67–88
Pérez A, Poza M, Aranda J, Latasa C, Medrano FJ, Tomás M, Romero A, Lasa I, Bou G et al (2012) Effect of the transcriptional activators SoxS, RobA and RamA on expression of the multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob Agents Chemother 56(12):6256–6266. https://doi.org/10.1128/AAC.01085-12
Pukatzki S, McAuley SB, Miyata ST (2009) The type VI secretion system: translocation of effectors and effector-domains. Curr Opin Microbiol 12:11–17
Ran S, Liu B, Jiang W, Sun Z, Liang J (2015) Transcriptome analysis of Enterococcus faecalis in response to alkaline stress. Front Microbiol 6:795. https://doi.org/10.3389/fmicb.2015.00795
Razzauti M, Galan M, Bernard M, Maman S, Klopp C, Charbonnel N, Taussat MV, Eloit M, Cosson JF (2015) A comparison between transcriptome sequencing and 16S metagenomics for detection of bacterial pathogens in wildlife. PLoS Negl Trop Dis 9:e0003929
Reeve WG, Tiwari RP, Kale NB, Dilworth MJ, Glenn AR (2002) actP controls copper homeostasis in R. leguminosarium bv. Viciae and Sinorhizobium meliloti preventing lowpH-induced copper toxicity. Mol Microbiol 43:981–91
Robinson MD, McCarthy DJ, Smyth GK (2010) EdgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140
Schwarz J, Schumacher K, Brameyer S, Jung K (2022) Bacterial battle against acidity. FEMS Microbiol Rev 46(6):fua037. https://doi.org/10.1093/femsre/fuac037
Šeputien V, Sužied K, Normark S, Melefors Ö, Sužied E (2004) Transcriptional analysis of the acid-inducible asr gene in enterobacteria. Res Microbiol 155:535–542
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideher T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Shrivastava S, Mande SS (2008) Identification and functional characterization of gene components of Type VI Secretion system in bacterial genomes. PLoS ONE 3:e2955
Singh RP, Jha PN (2018) Priming with ACC-utilizing bacterium attenuated copper toxicity, improved oxidative stress tolerance, and increased phytoextraction capacity in wheat. Environ Sci Pollut Res 25(33):33755–33767
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P et al (2017) The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res 45:D362–D368
Takada H, Morita M, Shiwa Y, Sugimoto R, Suzuki S, Kawamura F, Yoshikawa H (2014) Cell motility and biofilm formation in Bacillus subtilis are affected by the ribosomal proteins, S11 and S21. Biosci Biotechnol Biochem 78:898–907
Tiwari RP, Reeve WG, Fenner BJ, Dilworth MJ, Glenn AR, Howieson JG (2004) Probing for pH-regulated proteins in Sinorhizobium medicae using proteomic analysis. J Mol Microbiol Biotechnol 7:140–147
Toesca I, Perard C, Bouvier J, Gutierrez C, Conter A (2001) The transcriptional activator NhaR is responsible for the osmotic induction of osmC (p1), a promoter of the stress-inducible gene osmC in Escherichia coli. Microbiol 147:2795–2803. https://doi.org/10.1099/00221287-147-10-2795
Tokuda H, Matsuyama SI (2004) Sorting of lipoproteins to the outer membrane in E. coli. Biochim Biophys Acta - Mol Cell Res 1693:5–13
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, SalzbergSL WBJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Vassileva V, Milanov G, Ignatov G, Nikolov B (1997) Effect of low pH on nitrogen fixation of common bean grown at various calcium and nitrate levels. J Plant Nutr 20:279–294
Vebø HC, Solheim M, Snipen L, Nes IF, Brede DA (2010) Comparative genomic analysis of pathogenic and probiotic Enterococcus faecalis isolates, and their transcriptional responses to growth in human urine. PLoS ONE 5:e12489
Von Uexkull HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Wei Y, Zeng X, Yuan Y, Jiang H, Zheng Y, Tan Y, Guo Z, Yang R, Zhou D, Jiang Y (2011) DNA microarray analysis of acid-responsive genes of Streptococcus suis serotype 2. Annals Microbiol 61:505–510
Weiss MS, Abele U, Weckesser J, Welte WU, Schiltz E, Schulz GE (1991) Molecular architecture and electrostatic properties of a bacterial porin. Science 254:1627–1630
Wittmann H (1982) Components of bacterial ribosomes. Ann Rev Biochem 51:155–183
Xu X, Chen J, Huang X, Feng S, Zhang X, She F, Wen Y (2021) The Role of a Dipeptide Transporter in the Virulence of Human Pathogen. Helicobacter Pylori Front Microbiol 12:633166
Yang K, Ren Z, Raushel FM, Zhang J (2016) Structures of the Carbon-Phosphorus Lyase Complex Reveal the Binding Mode of the NBD-like PhnK. Structure 24:37–42. https://doi.org/10.1016/j.str.2015.11.009
Yang Q, Yang Y, Tang Y, Wang X, Chen Y, Shen W, Zhan Y, Gao J, Wu B, He M et al (2020) Development and characterization of acidic-pH-tolerant mutants of Zymomonas mobilis through adaptation and next-generation sequencing-based genome resequencing and RNASeq. Biotechnol Biofuels 13:144. https://doi.org/10.1186/s13068-020-01781-1
Yildiz FH, Schoolnik GK (1999) Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA 96:4028–4033
Yokota N, Kuroda T, Matsuyama SI, Tokuda H (1999) Characterization of the LolA-LolB system as the general lipoprotein localization mechanism of Escherichia coli. J Biol Chem 274:30995–30999
Yu KW, Xue P, Fu Y, Yang L (2021) T6SS mediated stress responses for bacterial environmental survival and host adaptation. Int J Mol Sci 22:478
Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JHD, Noller H (2001) Crystal structure of the ribosome at 5.5 Å resolution. Science 292:883–896
Zhang W, Wang Y, Song Y, Wang T, Xu S, Peng Z, Lin X, Zhang L, Shen X (2013) A type VI secretion system regulated by OmpR in Yersinia pseudotuberculosis functions to maintain intracellular pH homeostasis. Environ Microbiol 15:557–569
Zhang L, Dan Song D, Wu Z (2021) Transcriptome analysis of Cyclocaryapaliurus favonoids regulation of diferently expressed genes in Enterococcus faecalis under low pH stress. Arch Microbiol 203:2147–2155
Zhen W, Li Yang, Xuezheng L (2017) Transcriptome analysis of the Antarctic psychrotrophic bacterium Psychrobacter sp. G in response to temperature stress Acta Oceanol. Sinica 36(2):78–87
Żmijewski MA, Kwiatkowska JM, Lipińska B (2004) Complementation studies of the DnaK–DnaJ–GrpE chaperone machineries from Vibrio harveyi and Escherichia coli, both in vivo and in vitro. Arch Microbiol 182:436–449
Acknowledgements
RPS acknowledges the Department of Biotechnology, Government of India for providing the Ramalingswami Re-entry Fellowship. The author acknowledges the Dept. of Bioengineering and Biotechnology, BIT Mesra for providing the infrastructure.
Funding
The work was supported by the Ramalingswami Re-entry Grant provided by Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Sequence submission
The RNA sequence of S-33 was submitted to NCBI under BioProject No. PRJNA953758.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, K., Sharma, P.K. & Singh, R.P. The transcriptome response of Enterobacter sp. S-33 is modulated by low pH-stress. Genes Genom 46, 671–687 (2024). https://doi.org/10.1007/s13258-024-01513-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-024-01513-x