Pediatric Drugs ( IF 3.7 ) Pub Date : 2023-07-21 , DOI: 10.1007/s40272-023-00581-y Claire Yang 1 , Natalie Rosenwasser 1, 2 , Xing Wang 2 , Zheng Xu 1 , Joshua Scheck 1 , Markus D Boos 3 , Deepti Gupta 3 , Heather A Brandling-Bennet 3 , Robert Sidbury 3 , Ramesh S Iyer 4 , Yongdong Zhao 1, 2
|
Background
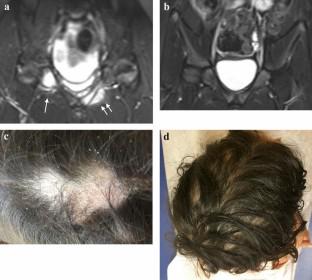
Chronic recurrent multifocal osteomyelitis (CRMO) is a rare autoinflammatory bone disease requiring immunosuppressive treatment in half of patients. Monoclonal tumor necrosis factor inhibitors (TNFi) are often used as effective second-line off-label therapies. However, paradoxical psoriasis can occur in a subset of patients exposed to monoclonal TNFi and can prompt conversion to alternate therapy if severe.
Objective
The aim of this study was to determine the efficacy and safety of golimumab, a fully humanized TNFi, in children with CRMO, including those who develop paradoxical psoriasis after exposure to other monoclonal TNFi.
Methods
A retrospective chart review was conducted of patients with CRMO who received golimumab in a single center between 01 June, 2018 and 31 December, 2020. Patients who were diagnosed before 21 years of age and followed up for CRMO at least once after receiving ≥ 3 months of golimumab were included. Extracted data included patient demographics, whole-body MRI lesion counts, clinically relevant data, laboratory results, patient-reported outcomes, and psoriasis burden. Linear mixed models with log-transformed outcomes were used to assess changes in the outcomes over time. The random effect is included in the model to account for the within-subject correlation of repeated measures. p-values and 95% confidence intervals were reported.
Results
Eighteen patients were included. Patients were observed for a median of 9.95 months [interquartile range 3.84–15.64]. The median age at the initiation of golimumab was 10.95 years [9.86–13.77] and the median duration of disease between the disease onset and the initiation of golimumab was 2.60 years [1.66–3.62]. Ten patients received golimumab via intravenous route and eight patients received golimumab via subcutaneous route. The median dose was 1.64 mg/kg/month [1.46, 2]. Fourteen patients were previously treated with disease-modifying antirheumatic drugs and 17 with other TNFi. Patients treated with golimumab showed significant improvement in median physician global assessment for CRMO from 2.00 [1.00–3.00] to 0.00 [0.00–0.25] by the fourth visit (p < 0.001), with median erythrocyte sedimentation rate (ESR) decreasing significantly from 12.00 [6.75–23.75] to 5.00 [3.00–10.00] by the fourth visit (p < 0.05). The median number of lesions on MRI decreased significantly from 3.50 [2.00–5.50] to 0.50 [0.00–4.25] lesions per patient (p < 0.01). Nine out of 12 patients who had previous paradoxical psoriasis associated with adalimumab or infliximab had persistent active psoriasis at study baseline. For patients with psoriasis at study baseline, the prevalence of psoriasis had decreased from 100% to approximately 50–57% at the following visits. Of the 18 patients initiated on golimumab in this study, there was only one new case of mild psoriasis in a patient with previously resolved infliximab-associated paradoxical psoriasis. No serious infections or adverse events were noted during the study. Two patients in the study showed clinical improvement with concomitant golimumab and ustekinumab with no reported adverse side effects or increased effects in these patients over a 16-month interval, showing that this combination can be safe and effective for children with CRMO.
Conclusion
In our experience, golimumab has been shown to be a safe and effective therapy for CRMO and demonstrated improvement in paradoxical psoriasis in many patients. Longer follow-up periods would be helpful to develop longer term outcomes data for patients with CRMO and overall paradoxical psoriasis risk.
中文翻译:

戈利木单抗治疗慢性复发性多灶性骨髓炎儿童:病例系列及文献回顾
背景
慢性复发性多灶性骨髓炎(CRMO)是一种罕见的自身炎症性骨病,一半患者需要免疫抑制治疗。单克隆肿瘤坏死因子抑制剂(TNFi)通常被用作有效的二线标签外治疗。然而,部分接触单克隆 TNFi 的患者可能会出现矛盾性银屑病,如果病情严重,可能会提示转为替代疗法。
客观的
本研究的目的是确定戈利木单抗(一种完全人源化的 TNFi)对 CRMO 儿童的疗效和安全性,包括那些在接触其他单克隆 TNFi 后出现矛盾性银屑病的儿童。
方法
对2018年6月1日至2020年12月31日期间在单中心接受戈利木单抗的CRMO患者进行回顾性图表审查。21岁之前诊断并在接受≥3个月后至少随访一次CRMO的患者戈利木单抗也被包括在内。提取的数据包括患者人口统计、全身 MRI 病变计数、临床相关数据、实验室结果、患者报告的结果和牛皮癣负担。使用具有对数转换结果的线性混合模型来评估结果随时间的变化。模型中包含随机效应,以解释重复测量的受试者内相关性。报告了p值和 95% 置信区间。
结果
包括十八名患者。患者的观察时间中位数为 9.95 个月 [四分位数范围 3.84–15.64]。开始使用戈利木单抗时的中位年龄为 10.95 岁 [9.86–13.77],从发病到开始使用戈利木单抗之间的中位疾病持续时间为 2.60 年 [1.66–3.62]。十名患者通过静脉途径接受戈利木单抗,八名患者通过皮下途径接受戈利木单抗。中位剂量为 1.64 mg/kg/月 [1.46, 2]。14 名患者之前接受过缓解病情的抗风湿药物治疗,17 名患者接受过其他 TNFi 治疗。接受戈利木单抗治疗的患者在第四次就诊时显示,医生对 CRMO 的中位整体评估从 2.00 [1.00–3.00] 显着改善至 0.00 [0.00–0.25] ( p < 0.001),中位红细胞沉降率 (ESR) 从 12.00 显着下降第四次就诊时,从 [6.75–23.75] 降至 5.00 [3.00–10.00] ( p < 0.05)。MRI 上的病灶中位数从每位患者 3.50 [2.00–5.50] 个病灶显着减少至 0.50 [0.00–4.25] 个病灶 ( p < 0.01)。先前患有与阿达木单抗或英夫利昔单抗相关的矛盾性银屑病的 12 名患者中,有 9 名在研究基线时患有持续性活动性银屑病。对于研究基线时患有银屑病的患者,在后续就诊时,银屑病患病率从 100% 降至约 50-57%。在本研究中开始接受戈利木单抗治疗的 18 名患者中,只有 1 名新发轻度银屑病病例,且该患者先前患有英夫利昔单抗相关的矛盾性银屑病。研究期间没有发现严重感染或不良事件。研究中的两名患者在联合使用戈利木单抗和乌特克单抗后表现出临床改善,并且在 16 个月的时间间隔内没有报告不良副作用或副作用增加,表明这种组合对于 CRMO 儿童来说是安全有效的。
结论
根据我们的经验,戈利木单抗已被证明是一种安全有效的 CRMO 疗法,并在许多患者的矛盾性银屑病中得到了改善。较长的随访期将有助于为 CRMO 患者和总体矛盾的银屑病风险开发长期结果数据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号