Journal of Pharmaceutical Innovation ( IF 2.6 ) Pub Date : 2023-11-30 , DOI: 10.1007/s12247-023-09801-x Yuanpeng Wang , Chensong Zhang , Shaobo Zhou , Liang Chu , Wei Fang , Jiachi Ma
|
Purpose
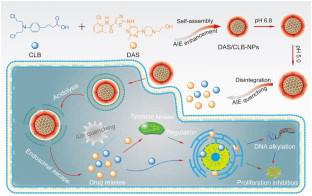
Dynamic carrier-free theranostic nanodrugs are in great demand, owing to their extraordinary high drug loading, enhanced targeting therapy, and panoramic tracking of the drug behaviors. Herein, this work highlights a successful development of pH-triggered dynamic carrier-free nanodrugs for precise tumoral targeting theragnostic, which are established through self-assembly between dasatinib (DAS) and chlorambucil (CLB).
Methods
The study has proved the structure, change in particle size and zeta potential, fluorescence transition, cellular uptake, cytotoxicity as well as biosafety of the carrier-free nanodrugs. The nanodrugs are characterized by Fourier transform infrared spectroscopy, 1H nuclear magnetic resonance, X-ray diffraction, Dynamic light scattering, and Microplate reader. Cellular uptake and cytotoxicity assay are conducted for free drugs and their nanodrugs using tumor cell lines including A549, HepG2, K562, and THP1. ICR mice are applied to evaluate the biosafety of nanodrugs.
Results
The introduction of CLB into DAS nanoparticles can successfully redshift the emission wavelength from 420 to 810 nm. Moreover, the nanodrugs exhibit a dynamic fluorescence intensity conversion via tumoral intracellular gradual quenching of Aggregation-induced emission (AIE). This characteristic is beneficial to the precise monitoring of tumoral intracellular drug behaviors. Furthermore, the nanodrugs show a small-to-large size transition from 175 nm to more than 500 nm in 12 h and surficial charge reversal from −2.3 mV to more than 0.2 mV by protonation at tumoral pHs. These superior properties facilitate the improved cellular uptake and synergistic cytotoxicity on various types of tumor cells.
Conclusion
The study shows that nanodrugs made of DAS and CLB that can self-assemble without carriers under different pH levels may be ready for testing in tumor targeting, and might someday be helpful for diagnosis and treatment in the future.
中文翻译:

由达沙替尼和苯丁酸氮芥自组装的 pH 触发动态无载体纳米药物,具有精确肿瘤靶向治疗诊断的潜力
目的
动态无载体治疗诊断纳米药物因其极高的载药量、增强的靶向治疗和药物行为的全景跟踪而受到巨大需求。在此,这项工作强调了pH触发的动态无载体纳米药物的成功开发,用于精确的肿瘤靶向治疗,这些药物是通过达沙替尼(DAS)和苯丁酸氮芥(CLB)之间的自组装而建立的。
方法
该研究证明了无载体纳米药物的结构、粒径和zeta电位的变化、荧光跃迁、细胞摄取、细胞毒性以及生物安全性。通过傅里叶变换红外光谱、1H核磁共振、X射线衍射、动态光散射和酶标仪对纳米药物进行了表征。使用 A549、HepG2、K562 和 THP1 等肿瘤细胞系对游离药物及其纳米药物进行细胞摄取和细胞毒性测定。ICR小鼠用于评估纳米药物的生物安全性。
结果
将CLB引入DAS纳米粒子可以成功地将发射波长从420 nm红移至810 nm。此外,纳米药物通过肿瘤细胞内聚集诱导发射(AIE)的逐渐猝灭表现出动态荧光强度转换。这一特性有利于肿瘤细胞内药物行为的精准监测。此外,纳米药物在 12 小时内表现出从 175 nm 到超过 500 nm 的小尺寸到大尺寸的转变,并且在肿瘤 pH 值下通过质子化,表面电荷从 -2.3 mV 反转到超过 0.2 mV。这些优异的特性有利于改善细胞摄取和对各种类型肿瘤细胞的协同细胞毒性。
结论
研究表明,由 DAS 和 CLB 制成的纳米药物可以在不同 pH 水平下无载体自组装,可能已准备好用于肿瘤靶向测试,并可能有助于未来的诊断和治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号