Biochemistry (Moscow) ( IF 2.8 ) Pub Date : 2023-12-27 , DOI: 10.1134/s0006297923120167 Alexander L. Ksenofontov , Ludmila A. Baratova , Pavel I. Semenyuk , Natalia V. Fedorova , Gennadii A. Badun
|
Abstract
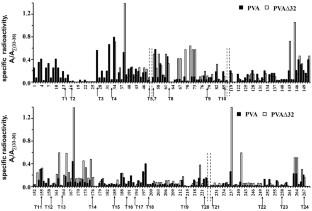
Coat proteins (CP) of the potato virus A virions (PVA) contain partially disordered N-terminal domains, which are necessary for performing vital functions of the virus. Comparative analysis of the structures of coat proteins (CPs) in the intact PVA virions and in the virus particles lacking N-terminal 32 amino acids (PVAΔ32) was carried out in this work based on the tritium planigraphy data. Using atomic-resolution structure of the potato virus Y potyvirus (PVY) protein, which is a homolog of the CP PVA, the available CP surfaces in the PVY virion were calculated and the areas of intersubunit/interhelix contacts were determined. For this purpose, the approach of Lee and Richards [Lee, B., and Richards, F. M. (1971) J. Mol. Biol., 55, 379-400] was used. Comparison of incorporation profiles of the tritium label in the intact and trypsin-degraded PVA∆32 revealed position of the ΔN-peptide shielding the surface domain (a.a. 66-73, 141-146) and the interhelix zone (a.a. 161-175) of the PVA CP. Presence of the channels/cavities was found in the virion, which turned out to be partially permeable to tritium atoms. Upon removal of the ∆N-peptide, decrease in the label incorporation within the virion (a.a. 184-200) was also observed, indicating possible structural transition leading to the virion compactization. Based on the obtained data, we can conclude that part of the surface ∆N-peptide is inserted between the coils of the virion helix thus increasing the helix pitch and providing greater flexibility of the virion, which is important for intercellular transport of the viruses in the plants.
中文翻译:

根据氚标记数据和计算机模拟有限原位蛋白水解后马铃薯病毒 A 病毒体结构的变化
摘要
马铃薯 A 病毒病毒体 (PVA) 的外壳蛋白 (CP) 含有部分无序的 N 末端结构域,这是执行病毒重要功能所必需的。本工作基于氚平面摄影数据,对完整 PVA 病毒体和 N 端 32 个氨基酸缺失的病毒颗粒 (PVAΔ32) 的外壳蛋白 (CP) 结构进行了比较分析。利用马铃薯病毒 Y 马铃薯病毒 (PVY) 蛋白(CP PVA 的同源物)的原子分辨率结构,计算了 PVY 病毒粒子中可用的 CP 表面,并确定了亚基间/螺旋间接触的面积。为此,Lee 和 Richards 的方法 [Lee, B., and Richards, F. M. (1971) J. Mol. 生物。,55,379-400 ]被使用。完整和胰蛋白酶降解的 PVAΔ32 中氚标记的掺入曲线的比较揭示了 ΔN 肽屏蔽表面结构域(氨基酸 66-73、141-146)和螺旋间区(氨基酸 161-175)的位置。 PVA CP。在病毒颗粒中发现了通道/空腔的存在,结果表明病毒颗粒对氚原子具有部分渗透性。去除 ΔN-肽后,还观察到病毒粒子 (aa 184-200) 内的标签掺入量减少,表明可能的结构转变导致病毒粒子压缩。根据获得的数据,我们可以得出结论,部分表面ΔN肽插入到病毒体螺旋的线圈之间,从而增加了螺旋螺距并为病毒体提供了更大的灵活性,这对于病毒的细胞间运输非常重要植物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号