Biochemistry (Moscow) ( IF 2.8 ) Pub Date : 2023-12-27 , DOI: 10.1134/s000629792312009x Anna A. Kudriaeva , Lyudmila A. Yakubova , George A. Saratov , Vasiliy I. Vladimirov , Valeriy M. Lipkin , Alexey A. Belogurov
|
Abstract
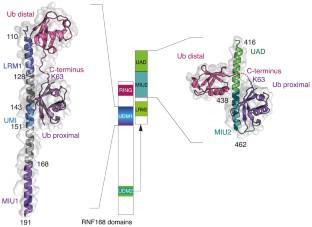
Genome stability is critical for normal functioning of cells, it depends on accuracy of DNA replication, chromosome segregation, and DNA repair. Cellular defense mechanisms against DNA damage are important for preventing cancer development and aging. The E3 ubiquitin ligase RNF168 of the RING superfamily is an essential component of the complex responsible for ubiquitination of the H2A/H2A.X histones near DNA double-strand breaks, which is a key step in attracting repair factors to the damage site. In this study, we unequivocally showed that RNF168 does not have the ability to directly distinguish architecture of polyubiquitin chains, except for the tropism of its two ubiquitin-binding domains UDM1/2 to K63 ubiquitin chains. Analysis of intracellular chromatosomal environment of the full-length RNF168 and its domains using the ligand-induced bioluminescence resonance energy transfer (BRET) revealed that the C-terminal part of UDM1 is associated with the K63 ubiquitin chains; RING and the N-terminal part of UDM2 are sterically close to the K63- and K48-ubiquitin chains, while the C-terminal part of UDM1 is co-localized with all possible ubiquitin variants. Our observations together with the available structural data suggest that the C-terminal part of UDM1 binds the K63 polyubiquitin chains on the linker histone H1; RING and the N-terminal part of UDM2 are located in the central part of nucleosome and sterically close to H1 and K48-ubiquitinated alternative substrates of RNF168, such as JMJD2A/B demethylases, while the C-terminal part of UDM1 is in the region of activated ubiquitin residue associated with E2 ubiquitin ligase, engaged by RNF168.
中文翻译:

E3 泛素连接酶 RNF168 染色体环境中泛素链的拓扑结构
摘要
基因组稳定性对于细胞的正常功能至关重要,它取决于 DNA 复制、染色体分离和 DNA 修复的准确性。针对 DNA 损伤的细胞防御机制对于预防癌症发展和衰老非常重要。RING 超家族的 E3 泛素连接酶 RNF168 是复合物的重要组成部分,负责 DNA 双链断裂附近的 H2A/H2A.X 组蛋白泛素化,这是将修复因子吸引到损伤位点的关键步骤。在这项研究中,我们明确表明,RNF168不具有直接区分多聚泛素链结构的能力,除了其两个泛素结合域UDM1/2至K63泛素链的向性外。使用配体诱导的生物发光共振能量转移(BRET)对全长 RNF168 及其结构域的细胞内染色体环境进行分析表明,UDM1 的 C 端部分与 K63 泛素链相关。RING 和 UDM2 的 N 端部分在空间上靠近 K63 和 K48 泛素链,而 UDM1 的 C 端部分与所有可能的泛素变体共定位。我们的观察结果与现有的结构数据表明,UDM1 的 C 末端部分与接头组蛋白 H1 上的 K63 多聚泛素链结合;RING 和 UDM2 的 N 端部分位于核小体的中心部分,在空间上靠近 RNF168 的 H1 和 K48 泛素化替代底物,例如 JMJD2A/B 去甲基化酶,而 UDM1 的 C 端部分位于该区域与 E2 泛素连接酶相关的活化泛素残基,由 RNF168 参与。



























 京公网安备 11010802027423号
京公网安备 11010802027423号