Indian Journal of Microbiology ( IF 3 ) Pub Date : 2024-04-07 , DOI: 10.1007/s12088-024-01252-3 Harpreet Kaur , Neelam Taneja
|
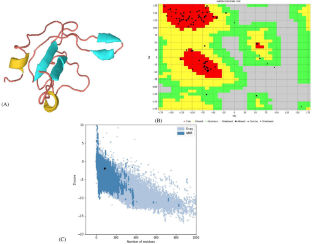
Escherichia coli (E. coli) is a gram-negative bacterial pathogen that poses a significant clinical and epidemiologic challenge. The selection pressure brought by the insufficient use of antibiotics has resulted in the emergence of multi-drug-resistant E. coli in the past ten years. Computational and bioinformatics methods for screening inhibitors have significantly contributed to discovering novel antibacterial agents. One possible target for novel anti-virulence drugs is motility. Motility inhibitors are generally effective at concentrations lower than those required for the antibacterial properties of traditional antibiotics, and they are likely to exert less selective pressure than current medicines. Motility may be essential for bacteria to survive, find nutrients, and escape unfavorable environments and biofilm formation. The FliN is a protein forming the bulk of the C ring of the flagella and is present in multiple copies (more than 100) in bacteria. Its absence in mammals makes it an attractive drug target for drug discovery. Two-thousand seven hundred seventy-eight natural compounds from the ZINC library were screened against FliN (PDB ID: 4YXB) using PyRx AutoDock Vina, and the top compounds were selected for secondary screening after sorting the results based on their binding energy. Based on interactional analysis, binding energy (− 7.78 kcal/mol), and inhibition constant (1.98 µM), ZINC000000619481 was the best inhibitor. This compound binds exactly as per the defined active site residues of the receptor protein. Also, molecular dynamics was performed. The eigenvalue of the selected complex was 1.241657e−05. There were no ADME properties outside of the specified range for the identified hit; it fitted exactly to the binding site of the FliN receptor well and was found to be stable in MD simulation studies. Further in vitro and in vivo studies are needed to confirm its anti-bacterial activity and use as a potential antimicrobial drug against urinary tract infections caused by E. coli.
中文翻译:

利用虚拟筛选和分子动力学模拟研究鉴定泌尿道致病性大肠杆菌鞭毛组装蛋白 FliN 抑制剂
大肠杆菌( E. coli ) 是一种革兰氏阴性细菌病原体,带来了重大的临床和流行病学挑战。抗生素使用不足带来的选择压力,导致近十年来多重耐药大肠杆菌的出现。用于筛选抑制剂的计算和生物信息学方法对发现新型抗菌剂做出了重大贡献。新型抗毒药物的一个可能目标是运动性。运动抑制剂通常在低于传统抗生素抗菌特性所需浓度的浓度下有效,并且它们产生的选择压力可能比现有药物小。运动性对于细菌生存、寻找营养、逃离不利环境和生物膜形成至关重要。 FliN 是一种构成鞭毛 C 环主体的蛋白质,在细菌中以多个拷贝(超过 100 个)存在。它在哺乳动物中不存在,使其成为药物发现中有吸引力的药物靶点。使用 PyRx AutoDock Vina 对 ZINC 库中的 2778 种天然化合物进行 FliN (PDB ID: 4YXB) 筛选,并根据结合能对结果进行排序后,选择排名靠前的化合物进行二次筛选。根据相互作用分析、结合能 (− 7.78 kcal/mol) 和抑制常数 (1.98 µM),ZINC000000619481 是最好的抑制剂。该化合物完全按照受体蛋白的指定活性位点残基进行结合。此外,还进行了分子动力学分析。所选复合体的特征值为 1.241657e−05。对于已识别的命中,不存在超出指定范围的 ADME 属性;它与 FliN 受体的结合位点完全吻合,并且在 MD 模拟研究中发现它是稳定的。需要进一步的体外和体内研究来证实其抗菌活性,并可作为对抗大肠杆菌引起的尿路感染的潜在抗菌药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号