当前位置:
X-MOL 学术
›
Mol. Biosyst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Screening for concanavalin A binders from a mannose-modified α-helix peptide phage library
Molecular BioSystems Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7mb00495h Iou Ven Chang 1, 2, 3, 4, 5 , Hiroshi Tsutsumi 5, 6, 7 , Hisakazu Mihara 5, 6, 7
Molecular BioSystems Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7mb00495h Iou Ven Chang 1, 2, 3, 4, 5 , Hiroshi Tsutsumi 5, 6, 7 , Hisakazu Mihara 5, 6, 7
Affiliation
|
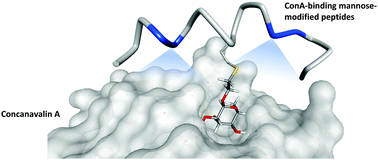
Mannose-modified lectin-binding peptides were obtained from an α-helical-designed peptide phage library. Concanavalin A (ConA) was used as a representative target protein for the lectin family. The identified glycopeptides could selectively bind to ConA with micromolar affinity. With these results, the methodologies described in this study will enhance the selection of saccharide-modified ligands through the synergistic effects of sugar and peptide units, with better specificity and affinity towards lectin proteins.
中文翻译:

从甘露糖修饰的α-螺旋肽噬菌体文库中筛选伴刀豆球蛋白A结合剂
从α-螺旋设计的肽噬菌体文库获得甘露糖修饰的凝集素结合肽。伴刀豆球蛋白A(ConA)被用作凝集素家族的代表性靶蛋白。鉴定出的糖肽可以微摩尔亲和力选择性结合ConA。有了这些结果,本研究中描述的方法将通过糖和肽单元的协同作用增强对糖修饰的配体的选择,并具有更好的对凝集素蛋白的特异性和亲和力。
更新日期:2017-10-25
中文翻译:

从甘露糖修饰的α-螺旋肽噬菌体文库中筛选伴刀豆球蛋白A结合剂
从α-螺旋设计的肽噬菌体文库获得甘露糖修饰的凝集素结合肽。伴刀豆球蛋白A(ConA)被用作凝集素家族的代表性靶蛋白。鉴定出的糖肽可以微摩尔亲和力选择性结合ConA。有了这些结果,本研究中描述的方法将通过糖和肽单元的协同作用增强对糖修饰的配体的选择,并具有更好的对凝集素蛋白的特异性和亲和力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号